当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemo- and Regioselective C−H Sulfidation of Indoles for the Synthesis of Tolylthioindoles under Metal-Free Conditions
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-11 , DOI: 10.1002/adsc.202200975 Nian Zheng 1 , Wei-Yu Shi 1 , Ya-Nan Ding 1 , Xue-yuan Liu 1 , Yong-Min Liang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-11 , DOI: 10.1002/adsc.202200975 Nian Zheng 1 , Wei-Yu Shi 1 , Ya-Nan Ding 1 , Xue-yuan Liu 1 , Yong-Min Liang 1
Affiliation
|
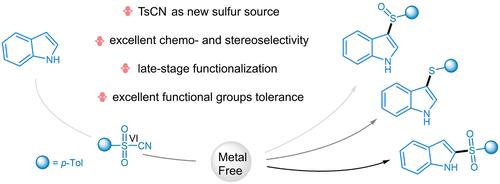
Controllable chemoselectivity and regioselectivity synthesis of 3-sulfinylated, 3-sulfenylated and 2-sulfonylated indoles via direct C−H functionalization are realized under metal-free conditions. In particular, the synthesis of 3-arylsulfinylindoles in high yields from a sulfone substrate in water without additives is reported. The process displays excellent functional groups compatibility. p-Toluenesulfonyl cyanide (TsCN) possesses a hexavalent sulfonyl group, as an odorless and easy-to-handle solid, which has been used as a new sulfur reagent through the cleavage of S−C and S=O bonds in our work.
中文翻译:

无金属条件下吲哚的化学和区域选择性 C−H 硫化合成甲苯基硫代吲哚
在无金属条件下,通过直接 C-H 官能化实现了 3-亚磺酰化、3-亚磺酰化和 2-磺酰化吲哚的可控化学选择性和区域选择性合成。特别是,报道了在没有添加剂的情况下,在水中从砜底物以高产率合成 3-芳基亚磺酰基吲哚。该过程显示出出色的官能团相容性。对甲苯磺酰氰 (TsCN) 具有六价磺酰基,是一种无味且易于处理的固体,在我们的工作中通过 S−C 和 S=O 键的裂解被用作新型硫试剂。
更新日期:2022-11-11
中文翻译:

无金属条件下吲哚的化学和区域选择性 C−H 硫化合成甲苯基硫代吲哚
在无金属条件下,通过直接 C-H 官能化实现了 3-亚磺酰化、3-亚磺酰化和 2-磺酰化吲哚的可控化学选择性和区域选择性合成。特别是,报道了在没有添加剂的情况下,在水中从砜底物以高产率合成 3-芳基亚磺酰基吲哚。该过程显示出出色的官能团相容性。对甲苯磺酰氰 (TsCN) 具有六价磺酰基,是一种无味且易于处理的固体,在我们的工作中通过 S−C 和 S=O 键的裂解被用作新型硫试剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号