当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective sulfonylation using sodium hydrogen sulfite, 4-substituted Hantzsch esters and 1-(arylethynyl)naphthalen-2-ols
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-11 , DOI: 10.1039/d2qo01654k Xinhua Wang 1 , Qiuping Ding 1 , Chenxi Yang 2 , Jianguo Yang 2 , Jie Wu 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-11 , DOI: 10.1039/d2qo01654k Xinhua Wang 1 , Qiuping Ding 1 , Chenxi Yang 2 , Jianguo Yang 2 , Jie Wu 2, 3, 4
Affiliation
|
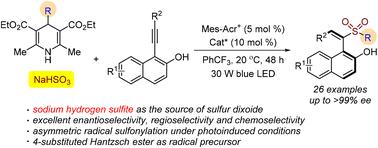
Synthesis of sulfonyl-containing axially chiral styrenes through a catalytic asymmetric three-component reaction of 4-substituted Hantzsch esters, NaHSO3 (sodium hydrogen sulfite), and 1-(arylethynyl)naphthalen-2-ols in the presence of a photocatalyst under visible light irradiation is reported. This transformation proceeds through a radical process under mild conditions, leading to axially chiral (S,E)-1-(1-(alkylsulfonyl)-2-arylvinyl)naphthalen-2-ols in good yields with excellent enantioselectivities. During the reaction process, excellent regioselectivity and chemoselectivity were observed as well. Notably, sodium hydrogen sulfite is used as the sulfur dioxide surrogate in this organocatalytic enantioselective radical sulfonylation under photoinduced conditions.
中文翻译:

使用亚硫酸氢钠、4-取代的 Hantzsch 酯和 1-(arylethynyl)naphthalen-2-ols 进行对映选择性磺酰化
在光催化剂存在下,4-取代的 Hantzsch 酯、NaHSO 3(亚硫酸氢钠)和 1-(arythynyl)naphthalen-2-ols通过催化不对称三组分反应合成含磺酰基的轴向手性苯乙烯报告了光照射。这种转变在温和条件下通过激进过程进行,导致轴向手性 ( S , E)-1-(1-(烷基磺酰基)-2-芳基乙烯基)萘-2-醇,产率高,对映体选择性好。在反应过程中,还观察到了优异的区域选择性和化学选择性。值得注意的是,亚硫酸氢钠在光诱导条件下的有机催化对映选择性自由基磺酰化中用作二氧化硫替代物。
更新日期:2022-11-11
中文翻译:

使用亚硫酸氢钠、4-取代的 Hantzsch 酯和 1-(arylethynyl)naphthalen-2-ols 进行对映选择性磺酰化
在光催化剂存在下,4-取代的 Hantzsch 酯、NaHSO 3(亚硫酸氢钠)和 1-(arythynyl)naphthalen-2-ols通过催化不对称三组分反应合成含磺酰基的轴向手性苯乙烯报告了光照射。这种转变在温和条件下通过激进过程进行,导致轴向手性 ( S , E)-1-(1-(烷基磺酰基)-2-芳基乙烯基)萘-2-醇,产率高,对映体选择性好。在反应过程中,还观察到了优异的区域选择性和化学选择性。值得注意的是,亚硫酸氢钠在光诱导条件下的有机催化对映选择性自由基磺酰化中用作二氧化硫替代物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号