当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Suzuki-Miyaura Coupling of Naphthalene-1,8-diaminato (dan)-Substituted Cyclopropylboron Compounds
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-10 , DOI: 10.1002/adsc.202201141 Mikinao Koishi 1 , Kazuki Tomota 1 , Masaaki Nakamoto 1 , Hiroto Yoshida 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-10 , DOI: 10.1002/adsc.202201141 Mikinao Koishi 1 , Kazuki Tomota 1 , Masaaki Nakamoto 1 , Hiroto Yoshida 1
Affiliation
|
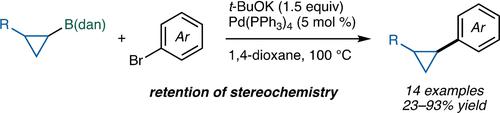
We herein describe the direct Suzuki-Miyaura coupling of dan-substituted, saturated organoboron compounds. Despite its Lewis acidity-diminished character of the boron center, cyclopropyl-B(dan) can be activated by t-BuOK to undergo transmetalation with a palladium complex. The increased s-character of the C−B(dan) bond as compared with other alkyl-B(dan) should be the key to the reaction.
中文翻译:

Naphthalene-1,8-diaminato (dan)-取代的环丙基硼化合物的直接 Suzuki-Miyaura 偶联
我们在此描述了丹取代的饱和有机硼化合物的直接 Suzuki-Miyaura 偶联。尽管硼中心具有路易斯酸度降低的特性,但环丙基-B(dan) 可以被t -BuOK 激活,与钯配合物进行金属转移。与其他烷基-B(dan)相比,C−B(dan)键的s-特征增加应该是反应的关键。
更新日期:2022-11-10
中文翻译:

Naphthalene-1,8-diaminato (dan)-取代的环丙基硼化合物的直接 Suzuki-Miyaura 偶联
我们在此描述了丹取代的饱和有机硼化合物的直接 Suzuki-Miyaura 偶联。尽管硼中心具有路易斯酸度降低的特性,但环丙基-B(dan) 可以被t -BuOK 激活,与钯配合物进行金属转移。与其他烷基-B(dan)相比,C−B(dan)键的s-特征增加应该是反应的关键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号