当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonlinear Effects in Asymmetric Catalysis by Design: Concept, Synthesis, and Applications
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-11-09 , DOI: 10.1021/acs.accounts.2c00557 Lena C Mayer 1 , Simone Heitsch 1 , Oliver Trapp 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-11-09 , DOI: 10.1021/acs.accounts.2c00557 Lena C Mayer 1 , Simone Heitsch 1 , Oliver Trapp 1
Affiliation
|
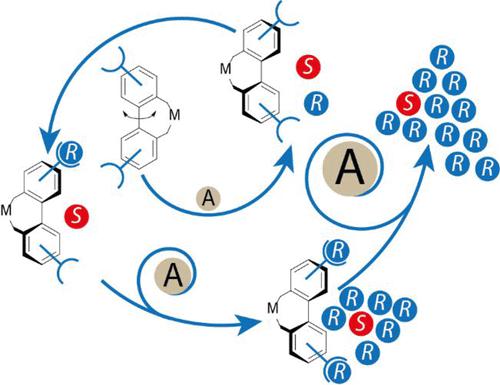
Asymmetric synthesis constitutes a key technology for the preparation of enantiomerically pure compounds as well as for the selective control of individual stereocenters in the synthesis of complex compounds. It is thus of extraordinary importance for the synthesis of chiral drugs, dietary supplements, flavors, and fragrances, as well as novel materials with tunable and reconfigurable chiroptical properties or the assembly of complex natural products. Typically, enantiomerically pure catalysts are used for this purpose. To prepare enantiomerically pure ligands or organocatalysts, one can make use of the natural chiral pool. Ligands and organocatalysts with an atropisomeric biphenyl and binaphthyl system have become popular, as they are configurationally stable and contain a C2-symmetric skeleton, which has been found to be particularly privileged. For catalysts with opposite configurations, both product enantiomers can be obtained. Configurationally flexible biphenyl systems initially appeared to be unsuitable for this purpose, as they racemize after successful enantiomer separation and thus are neither storable nor afford a reproducible enantioselectivity. However, there are strategies that exploit the dynamics of such ligands to stereoconvergently enrich one of the catalyst enantiomers. This can be achieved, for example, by coordinating an enantiomerically pure additive to a ligand–metal complex, which results in deracemization of the configurationally flexible biphenyl system, thereby enriching the thermodynamically preferred diastereomer. In this Account, we present our strategy to design stereochemically flexible catalysts that combine the properties of supramolecular recognition, stereoconvergent alignment, and catalysis. Such systems are capable to recognize the chirality of the target product, leading to an increase in enantioselectivity during asymmetric catalysis. We have systematically developed and investigated these smart catalyst systems and have found ways to specifically design and synthesize them for various applications. In addition to (i) reaction product-induced chiral amplification, we have developed systems with (ii) intermolecular and (iii) intramolecular recognition, and successfully applied them in asymmetric catalysis. Our results pave the way for new applications such as temperature-controlled enantioselectivity, controlled inversion of enantioselectivity with the same chirality of the recognition unit, generation of positive nonlinear effects, and targeted design of autocatalytic systems through dynamic formation of transient catalysts. Understanding such systems is of enormous importance for catalytic processes leading to symmetry breaking and amplification of small imbalances of enantiomers and offer a possible explanation of homochirality of biological systems. In addition, we are learning how to target supramolecular interactions to enhance enantioselectivities in asymmetric catalysis through secondary double stereocontrol. Configurationally flexible catalysts will enable future resource-efficient development of asymmetric syntheses, as enantioselectivities can be fully switched by stereoselective alignment of the stereochemically flexible ligand core on demand.
中文翻译:

设计不对称催化中的非线性效应:概念、合成和应用
不对称合成是制备对映体纯化合物以及在复杂化合物合成中选择性控制单个立体中心的关键技术。因此,它对于手性药物、膳食补充剂、香料和香料的合成,以及具有可调谐和可重构手性光学特性的新材料或复杂天然产物的组装具有极其重要的意义。通常,对映体纯催化剂用于此目的。要制备对映体纯的配体或有机催化剂,可以利用天然手性池。具有阻转异构联苯和联萘系统的配体和有机催化剂已变得流行,因为它们构型稳定且含有C2-对称骨架,已被发现特别有特权。对于具有相反构型的催化剂,可以获得两种产物对映异构体。构型灵活的联苯系统最初似乎不适合此目的,因为它们在对映异构体成功分离后会发生外消旋,因此既不能储存也不能提供可重现的对映选择性。然而,有一些策略利用此类配体的动力学来立体聚合地富集其中一种催化剂对映体。例如,这可以通过将对映体纯的添加剂与配体-金属络合物配位来实现,这会导致构型灵活的联苯系统发生去外消旋化,从而丰富热力学上优选的非对映异构体。在这个帐户中,我们提出了设计立体化学柔性催化剂的策略,这些催化剂结合了超分子识别、立体会聚排列和催化的特性。这样的系统能够识别目标产物的手性,导致不对称催化过程中对映选择性的增加。我们系统地开发和研究了这些智能催化剂系统,并找到了针对各种应用专门设计和合成它们的方法。除了 (i) 反应产物诱导的手性放大,我们还开发了具有 (ii) 分子间和 (iii) 分子内识别的系统,并成功地将它们应用于不对称催化。我们的结果为温度控制对映选择性等新应用铺平了道路,具有识别单元相同手性的对映选择性的受控反转,正非线性效应的产生,以及通过瞬态催化剂的动态形成来有针对性地设计自催化系统。了解此类系统对于导致对称性破坏和对映异构体的小失衡放大的催化过程非常重要,并为生物系统的同手性提供了可能的解释。此外,我们正在学习如何靶向超分子相互作用,以通过二次双立体控制增强不对称催化中的对映选择性。配置灵活的催化剂将使未来不对称合成的资源高效开发成为可能,
更新日期:2022-11-09
中文翻译:

设计不对称催化中的非线性效应:概念、合成和应用
不对称合成是制备对映体纯化合物以及在复杂化合物合成中选择性控制单个立体中心的关键技术。因此,它对于手性药物、膳食补充剂、香料和香料的合成,以及具有可调谐和可重构手性光学特性的新材料或复杂天然产物的组装具有极其重要的意义。通常,对映体纯催化剂用于此目的。要制备对映体纯的配体或有机催化剂,可以利用天然手性池。具有阻转异构联苯和联萘系统的配体和有机催化剂已变得流行,因为它们构型稳定且含有C2-对称骨架,已被发现特别有特权。对于具有相反构型的催化剂,可以获得两种产物对映异构体。构型灵活的联苯系统最初似乎不适合此目的,因为它们在对映异构体成功分离后会发生外消旋,因此既不能储存也不能提供可重现的对映选择性。然而,有一些策略利用此类配体的动力学来立体聚合地富集其中一种催化剂对映体。例如,这可以通过将对映体纯的添加剂与配体-金属络合物配位来实现,这会导致构型灵活的联苯系统发生去外消旋化,从而丰富热力学上优选的非对映异构体。在这个帐户中,我们提出了设计立体化学柔性催化剂的策略,这些催化剂结合了超分子识别、立体会聚排列和催化的特性。这样的系统能够识别目标产物的手性,导致不对称催化过程中对映选择性的增加。我们系统地开发和研究了这些智能催化剂系统,并找到了针对各种应用专门设计和合成它们的方法。除了 (i) 反应产物诱导的手性放大,我们还开发了具有 (ii) 分子间和 (iii) 分子内识别的系统,并成功地将它们应用于不对称催化。我们的结果为温度控制对映选择性等新应用铺平了道路,具有识别单元相同手性的对映选择性的受控反转,正非线性效应的产生,以及通过瞬态催化剂的动态形成来有针对性地设计自催化系统。了解此类系统对于导致对称性破坏和对映异构体的小失衡放大的催化过程非常重要,并为生物系统的同手性提供了可能的解释。此外,我们正在学习如何靶向超分子相互作用,以通过二次双立体控制增强不对称催化中的对映选择性。配置灵活的催化剂将使未来不对称合成的资源高效开发成为可能,











































 京公网安备 11010802027423号
京公网安备 11010802027423号