当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1,2,3,4-Tetrahydroisoquinolines via Palladium-Catalyzed Cyclization of N-Tosylhydrazones with ortho-Bromophenethyl Tosylamides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-08 , DOI: 10.1002/adsc.202200931 Jieming Zhang 1 , Zhan-Ming Zhang 2 , Qi Wu 3 , Junliang Zhang 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-08 , DOI: 10.1002/adsc.202200931 Jieming Zhang 1 , Zhan-Ming Zhang 2 , Qi Wu 3 , Junliang Zhang 2
Affiliation
|
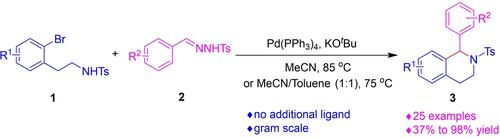
A Pd-catalyzed synthesis of 1,2,3,4-tetrahydroisoquinoline (THIQ) via cyclization of ortho-bromophenethyl tosylamides with aryl N-tosylhydrazones in the presence of base was developed. The reaction is believed to proceed via the oxidative addition of palladium with ortho-bromophenethyl tosylamides, followed by carbene insertion and cyclization. The carbene species were generated in-situ from the aryl N-tosylhydrazones and KOtBu.
中文翻译:

钯催化 N-Tosylhydrazones 与 ortho-Bromophenethyl Tosylamides 环化合成 1,2,3,4-四氢异喹啉
开发了一种 Pd 催化合成 1,2,3,4-四氢异喹啉 (THIQ),方法是在碱存在下邻溴苯乙基甲苯磺酰胺与芳基N-甲苯磺酰腙环化。据信该反应是通过钯与邻-溴苯乙基甲苯磺酰胺的氧化加成反应进行的,然后是卡宾插入和环化。卡宾物种由芳基N-甲苯磺酰腙和 KO t Bu 原位生成。
更新日期:2022-11-08
中文翻译:

钯催化 N-Tosylhydrazones 与 ortho-Bromophenethyl Tosylamides 环化合成 1,2,3,4-四氢异喹啉
开发了一种 Pd 催化合成 1,2,3,4-四氢异喹啉 (THIQ),方法是在碱存在下邻溴苯乙基甲苯磺酰胺与芳基N-甲苯磺酰腙环化。据信该反应是通过钯与邻-溴苯乙基甲苯磺酰胺的氧化加成反应进行的,然后是卡宾插入和环化。卡宾物种由芳基N-甲苯磺酰腙和 KO t Bu 原位生成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号