当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
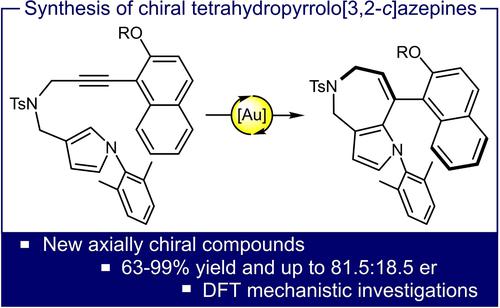
Atroposelective Synthesis of Tetrahydropyrrolo[3,2-c]azepine Derivatives Through Gold(I)-Catalyzed Hydroarylation
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-08 , DOI: 10.1002/adsc.202200828 Quentin Gaignard-Gaillard 1 , Xu Han 2 , Aurélien Alix 1 , Christophe Bour 3 , Regis Guillot 4 , vincent gandon 5 , Arnaud Voituriez 6
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-08 , DOI: 10.1002/adsc.202200828 Quentin Gaignard-Gaillard 1 , Xu Han 2 , Aurélien Alix 1 , Christophe Bour 3 , Regis Guillot 4 , vincent gandon 5 , Arnaud Voituriez 6
Affiliation
|
A series of enantioenriched substituted tetrahydropyrrolo[3,2-c]azepine products possessing an axial chirality were synthesized in 63–99% yield through a gold(I)-catalyzed hydroarylation. DFT calculations rationalized the reactivity observed for the formation of such complex molecular frameworks and guided our choice in the pyrrole N-substituent to isolate configurationally stable compounds.
中文翻译:

金(I)催化加氢芳基化反应选择性合成四氢吡咯并[3,2-c]氮杂衍生物
通过金 (I) 催化的加氢芳基化反应,合成了一系列具有轴向手性的对映体富集取代四氢吡咯并[3,2- c ]氮杂产品,产率为 63-99%。DFT 计算使观察到的形成此类复杂分子框架的反应性合理化,并指导我们选择吡咯N-取代基以分离构型稳定的化合物。
更新日期:2022-11-08
中文翻译:

金(I)催化加氢芳基化反应选择性合成四氢吡咯并[3,2-c]氮杂衍生物
通过金 (I) 催化的加氢芳基化反应,合成了一系列具有轴向手性的对映体富集取代四氢吡咯并[3,2- c ]氮杂产品,产率为 63-99%。DFT 计算使观察到的形成此类复杂分子框架的反应性合理化,并指导我们选择吡咯N-取代基以分离构型稳定的化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号