当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual nickel/photoredox catalyzed carboxylation of C(sp2)-halides with formate
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-08 , DOI: 10.1039/d2qo01361d Ming-Chen Fu 1, 2 , Jia-Xin Wang 2 , Wei Ge 2 , Fang-Ming Du 1 , Yao Fu 2
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-08 , DOI: 10.1039/d2qo01361d Ming-Chen Fu 1, 2 , Jia-Xin Wang 2 , Wei Ge 2 , Fang-Ming Du 1 , Yao Fu 2
Affiliation
|
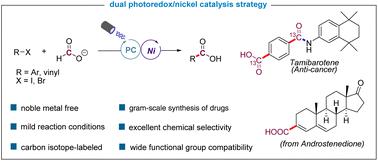
Herein, we report an efficient and practical protocol for dehalocarboxylation of C(sp2)-halides with formate by the engagement of a CO2 radical anion in a nickel-mediated bond-forming process. A wide variety of aryl iodides, aryl bromides, and alkenyl bromides bearing a diverse set of functional groups underwent the reaction smoothly through visible-light photoredox nickel dual catalysis. The synthesis of several 13C-labeled drug intermediates and the gram-scale synthesis of commercial drugs highlight the synthetic value of the approach in drug discovery settings.
中文翻译:

双镍/光氧化还原催化 C(sp2)-卤化物与甲酸盐的羧化
在此,我们报告了一种有效且实用的方案,通过在镍介导的键形成过程中参与 CO 2自由基阴离子,使 C(sp 2 )-卤化物与甲酸盐脱卤羧化。具有不同官能团的各种芳基碘化物、芳基溴化物和烯基溴化物通过可见光光氧化还原镍双催化顺利进行反应。几种13 C 标记的药物中间体的合成和商业药物的克级合成突出了该方法在药物发现环境中的合成价值。
更新日期:2022-11-08
中文翻译:

双镍/光氧化还原催化 C(sp2)-卤化物与甲酸盐的羧化
在此,我们报告了一种有效且实用的方案,通过在镍介导的键形成过程中参与 CO 2自由基阴离子,使 C(sp 2 )-卤化物与甲酸盐脱卤羧化。具有不同官能团的各种芳基碘化物、芳基溴化物和烯基溴化物通过可见光光氧化还原镍双催化顺利进行反应。几种13 C 标记的药物中间体的合成和商业药物的克级合成突出了该方法在药物发现环境中的合成价值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号