Energy Conversion and Management ( IF 9.9 ) Pub Date : 2022-11-08 , DOI: 10.1016/j.enconman.2022.116421 T. Shi , H.J. Xu , H.B. Ke , C.Y. Zhao
|
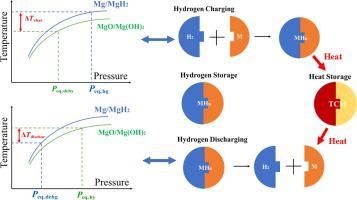
Metal hydride is a promising alternative for hydrogen storage. A novel metal hydride reactor coupled with thermochemical heat storage material is proposed to reduce the energy loss. The hydrogen charging and discharging processes are numerically and analytically investigated in this paper. Hydrogen is stored in a compacted metal hydride disk, so the reaction characteristics under low permeability are studied. The dehydration kinetic equation has been re-established to determine the equilibrium temperature at a corresponding pressure. The reactor takes 139 min for the hydrogen charging process and takes 512.8 min for the hydrogen discharging process. The reaction characteristics are found to be affected by heat transfer and gas pressure at the permeability of . The analytical model is found to be only applicable to the prediction of reaction time with high permeability of hydrogen in metal hydride. The thermal management of thermochemical heat storage materials has been proven to be feasible. By increasing the hydration equilibrium temperature from 321.8 °C to 350 °C, the reaction time can be decreased to 43.1 %. By decreasing the dehydration equilibrium temperature from 300 °C to 270 °C, the reaction time can be decreased to 84.5 %. To enhance the heat transfer, meal foam is added to the thermochemical heat storage material. Adding metal foam can effectively shorten the reaction time, but will reduce the hydrogen production. A metal foam with a porosity of 0.96 will reduce the production of hydrogen by 3 %.
中文翻译:

金属氢化物反应器与热化学储热材料相结合的储氢充放电热传输
金属氢化物是一种很有前途的储氢替代品。提出了一种与热化学储热材料相结合的新型金属氢化物反应器,以减少能量损失。本文对充放电过程进行了数值分析和分析研究。氢气储存在压实的金属氢化物盘中,因此研究了低渗透率下的反应特性。重新建立了脱水动力学方程以确定相应压力下的平衡温度。反应器充氢过程耗时139 min,放氢过程耗时512.8 min。发现反应特性受热传递和气体压力的影响。. 发现该解析模型仅适用于金属氢化物中氢的高渗透性反应时间的预测。热化学储热材料的热管理已被证明是可行的。通过将水合平衡温度从 321.8 °C 提高到 350 °C,反应时间可以减少到 43.1%。通过将脱水平衡温度从 300 °C 降低到 270 °C,反应时间可以降低到 84.5%。为了增强热传递,在热化学储热材料中添加了粗粉泡沫。添加泡沫金属可有效缩短反应时间,但会减少氢气的产生。孔隙率为 0.96 的金属泡沫将减少 3% 的氢气产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号