Journal of Advanced Research ( IF 11.4 ) Pub Date : 2022-11-05 , DOI: 10.1016/j.jare.2022.10.018 Cailing Gan 1 , Yan Wang 1 , Zhongzheng Xiang 1 , Hongyao Liu 1 , Zui Tan 1 , Yuting Xie 1 , Yuqin Yao 2 , Liang Ouyang 1 , Changyang Gong 1 , Tinghong Ye 1
|
Introduction
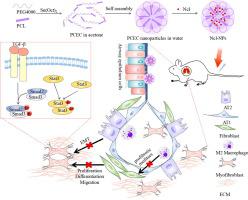
Idiopathic pulmonary fibrosis (IPF), a life-threatening interstitial lung disease, is characterized by excessive activation and proliferation of fibroblasts and epithelial-mesenchymal transition (EMT) of alveolar epithelial cells (AEC) accompanied by a large amount of extracellular matrix aggregation. There are no therapies to reverse pulmonary fibrosis, and nintedanib and pirfenidone could only slow down the decline of lung function of IPF patients and delay their survival time. Niclosamide (Ncl) is an antihelminthic drug approved by FDA, which has been reported to have pleiotropic pharmacological activities in recent years, but it’s almost complete insolubility in water limits its clinical application.
Objectives
To improve the water solubility of Ncl, explore its ability to reverse BLM-induced pulmonary fibrosis and its specific mechanism of action.
Methods
The Niclosamide-loaded nanoparticles (Ncl-NPs) were formed by emulsification solvent evaporation method. A mouse model induced by bleomycin (BLM) was established to evaluate its effects and mechanisms of inhibiting and reversing fibrosis in vivo. The cell models treated by transforming growth factor-β1 (TGF-β1) were used to examine the mechanism of Ncl-NPs inhibiting fibrosis in vitro. Flow cytometry, IHC, IL-4-induced macrophage model and co-culture system were used to assess the effect of Ncl-NPs on M2 polarization of macrophages.
Results
The Ncl-NPs improved the poor water solubility of Ncl. The lower dose of Ncl-NPs (2.5 mg/kg) showed the same effect of reversing established pulmonary fibrosis as free Ncl (5 mg/kg). Mechanistic studies revealed that Ncl-NPs blocked TGF-β/Smad and signaling transducer and activator of transcription 3 (Stat3) signaling pathways and inhibited the M2 polarization of macrophages. Additionally, H&E staining of the tissues initially showed the safety of Ncl-NPs.
Conclusion
These results indicate Ncl-NPs may serve as a new idea for the treatment of pulmonary fibrosis.
中文翻译:

负载氯硝柳胺的纳米粒子(Ncl-NPs)在体内和体外逆转肺纤维化
介绍
特发性肺纤维化(IPF)是一种危及生命的间质性肺疾病,其特征是成纤维细胞过度活化和增殖以及肺泡上皮细胞(AEC)的上皮间质转化(EMT)并伴有大量细胞外基质聚集。目前尚无逆转肺纤维化的治疗方法,尼达尼布和吡非尼酮只能减缓IPF患者肺功能的衰退,延长患者的生存时间。氯硝柳胺(Ncl)是FDA批准的抗蠕虫药,近年来报道其具有多效药理活性,但其几乎完全不溶于水,限制了其临床应用。
目标
为了提高Ncl的水溶性,探讨其逆转BLM所致肺纤维化的能力及其具体作用机制。
方法
采用乳化溶剂蒸发法制备了氯硝柳胺纳米颗粒(Ncl-NPs)。建立博莱霉素(BLM)诱导的小鼠模型,以评价其体内抑制和逆转纤维化的作用和机制。利用转化生长因子-β1(TGF-β1)处理的细胞模型来研究Ncl-NPs体外抑制纤维化的机制。采用流式细胞术、IHC、IL-4诱导的巨噬细胞模型和共培养系统评估Ncl-NPs对巨噬细胞M2极化的影响。
结果
Ncl-NPs改善了Ncl较差的水溶性。较低剂量的 Ncl-NP(2.5 毫克/千克)显示出与游离 Ncl(5 毫克/千克)相同的逆转已形成的肺纤维化的效果。机制研究表明,Ncl-NPs 阻断 TGF-β/Smad 和信号转导子和转录激活子 3 (Stat3) 信号通路,并抑制巨噬细胞的 M2 极化。此外,组织的 H&E 染色初步显示了 Ncl-NP 的安全性。
结论
这些结果表明Ncl-NPs可能成为治疗肺纤维化的新思路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号