当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silver-catalysed [3 + 2] annulation reaction of aryldiazonium salts with allenes enabled by boronate direction
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-09 , DOI: 10.1039/d2qo01585d Xing Peng 1 , Meng-Meng Zheng 2 , Pei Qin 1 , Xiao-Song Xue 2, 3 , Fa-Guang Zhang 1 , Jun-An Ma 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-09 , DOI: 10.1039/d2qo01585d Xing Peng 1 , Meng-Meng Zheng 2 , Pei Qin 1 , Xiao-Song Xue 2, 3 , Fa-Guang Zhang 1 , Jun-An Ma 1
Affiliation
|
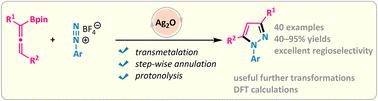
Allenes are a unique type of powerful synthon used for the construction of various carbocycles and heterocycles, but their annulation reactions with N–N triple bond electrophiles have not been disclosed. Here we report an efficient silver-catalysed boronate-directed [3 + 2] annulation reaction of aryl diazonium salts with allenylboronates. This transformation offers unprecedented access to a wide scope of N1-aryl-1H-pyrazoles with high regioselectivities under mild conditions. Preliminary experimental and computational studies support a transmetalation/stepwise cycloaddition/pyrazolyl silver hydrolysis pathway involving propargyl silver species as the key intermediate in enabling reactivity and controlling regioselectivity.
中文翻译:

银催化的芳基重氮盐与丙二烯的[3+2]环化反应由硼酸盐方向引发
丙二烯是一种独特类型的强大合成子,用于构建各种碳环和杂环,但它们与 N-N 三键亲电子试剂的环化反应尚未公开。在这里,我们报告了芳基重氮盐与丙二烯基硼酸盐的高效银催化硼酸盐导向 [3 + 2] 环化反应。这种转变为在温和条件下具有高区域选择性的范围广泛的N 1 -aryl-1 H -吡唑提供了前所未有的途径。初步实验和计算研究支持转金属化/逐步环加成/吡唑基银水解途径,涉及炔丙基银物种作为实现反应性和控制区域选择性的关键中间体。
更新日期:2022-11-09
中文翻译:

银催化的芳基重氮盐与丙二烯的[3+2]环化反应由硼酸盐方向引发
丙二烯是一种独特类型的强大合成子,用于构建各种碳环和杂环,但它们与 N-N 三键亲电子试剂的环化反应尚未公开。在这里,我们报告了芳基重氮盐与丙二烯基硼酸盐的高效银催化硼酸盐导向 [3 + 2] 环化反应。这种转变为在温和条件下具有高区域选择性的范围广泛的N 1 -aryl-1 H -吡唑提供了前所未有的途径。初步实验和计算研究支持转金属化/逐步环加成/吡唑基银水解途径,涉及炔丙基银物种作为实现反应性和控制区域选择性的关键中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号