当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A substrate-controlled Ru(II)-catalyzed C–H activation/[5 + 2] annulation cascade and unusual acyl migration to synthesize diverse indoline scaffolds
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-08 , DOI: 10.1039/d2qo01529c Miao Zheng 1, 2, 3 , Jianhui Zhou 2, 3 , Feifei Fang 2, 3 , Zichao Xu 2, 3 , Fuqiang Zheng 2, 3 , Zhidong Jiang 2, 3 , Tao Liu 1, 2, 3 , Hong Liu 2, 3, 4 , Linxiang Zhao 1 , Yu Zhou 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-08 , DOI: 10.1039/d2qo01529c Miao Zheng 1, 2, 3 , Jianhui Zhou 2, 3 , Feifei Fang 2, 3 , Zichao Xu 2, 3 , Fuqiang Zheng 2, 3 , Zhidong Jiang 2, 3 , Tao Liu 1, 2, 3 , Hong Liu 2, 3, 4 , Linxiang Zhao 1 , Yu Zhou 2, 3, 4
Affiliation
|
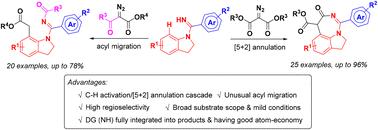
A substrate-controlled Ru(II)-catalyzed C–H activation/[5 + 2] annulation cascade and unusual acyl migration to synthesize diverse indoline scaffolds are reported. The most pronounced advantage is that this strategy can offer a highly selective C–H activation of N-benzamidine indoline with two reaction sites prone to C–H activation to selectively prepare a 1,7-fused indoline scaffold and a C7-alkylated/N′-acylated indoline ring by varying its diazo synthons. More importantly, the NH directing group of N-aryl benzamidines is fully utilized and atom-economically integrated into the target products with a good yield and broad substrate scope.
中文翻译:

底物控制的 Ru(II) 催化的 C-H 活化/[5 + 2] 环化级联和不寻常的酰基迁移以合成不同的二氢吲哚支架
报道了底物控制的 Ru( II ) 催化的 C-H 活化/[5 + 2] 环化级联和不寻常的酰基迁移以合成不同的二氢吲哚支架。最显着的优点是,该策略可以提供高选择性的N-苯甲脒二氢吲哚的 C-H 活化,具有两个易于 C-H 活化的反应位点,以选择性地制备 1,7-融合的二氢吲哚支架和 C7-烷基化/ N '-酰化二氢吲哚环通过改变其重氮合成子。更重要的是, N-芳基苯甲脒的NH导向基团得到充分利用,并以原子经济方式整合到目标产物中,产率高,底物范围广。
更新日期:2022-11-08
中文翻译:

底物控制的 Ru(II) 催化的 C-H 活化/[5 + 2] 环化级联和不寻常的酰基迁移以合成不同的二氢吲哚支架
报道了底物控制的 Ru( II ) 催化的 C-H 活化/[5 + 2] 环化级联和不寻常的酰基迁移以合成不同的二氢吲哚支架。最显着的优点是,该策略可以提供高选择性的N-苯甲脒二氢吲哚的 C-H 活化,具有两个易于 C-H 活化的反应位点,以选择性地制备 1,7-融合的二氢吲哚支架和 C7-烷基化/ N '-酰化二氢吲哚环通过改变其重氮合成子。更重要的是, N-芳基苯甲脒的NH导向基团得到充分利用,并以原子经济方式整合到目标产物中,产率高,底物范围广。











































 京公网安备 11010802027423号
京公网安备 11010802027423号