当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Do Go Chasing Waterfalls: Enoyl Reductase (FabI) in Complex with Inhibitors Stabilizes the Tetrameric Structure and Opens Water Channels
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-11-07 , DOI: 10.1021/acs.jcim.2c01178 Vinicius Gonçalves Maltarollo 1 , Ekaterina Shevchenko 2, 3, 4 , Igor Daniel de Miranda Lima 5 , Elio A Cino 5 , Glaucio Monteiro Ferreira 6 , Antti Poso 2, 3, 4, 7 , Thales Kronenberger 2, 3, 4, 7
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-11-07 , DOI: 10.1021/acs.jcim.2c01178 Vinicius Gonçalves Maltarollo 1 , Ekaterina Shevchenko 2, 3, 4 , Igor Daniel de Miranda Lima 5 , Elio A Cino 5 , Glaucio Monteiro Ferreira 6 , Antti Poso 2, 3, 4, 7 , Thales Kronenberger 2, 3, 4, 7
Affiliation
|
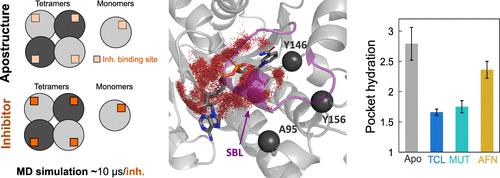
The enzyme enoyl-ACP reductase (FabI) is the limiting step of the membrane’s fatty acid biosynthesis in bacteria and a druggable target for novel antibacterial agents. The FabI active form is a homotetramer, which displays the highest affinity to inhibitors. Herein, molecular dynamics studies were carried out using the structure of FabI in complex with known inhibitors to investigate their effects on tetramerization. Our results suggest that multimerization is essential for the integrity of the catalytic site and that inhibitor binding enables the multimerization by stabilizing the substrate binding loop (SBL, L:195-200) coupled with changes in the H4/5 (QR interface). We also observed that AFN-1252 (naphtpyridinone derivative) promotes unique conformational changes affecting monomer–monomer interfaces. These changes are induced by AFN-1252 interaction with key residues in the binding sites (Ala95, Tyr146, and Tyr156). In addition, the analysis of water trajectories indicated that AFN-1252 complexes allow more water molecules to enter the binding site than triclosan and MUT056399 complexes. FabI–AFN-1252 simulations show accumulation of water molecules near the Tyr146/147 pocket, which can become a hotspot to the design of novel FabI inhibitors.
中文翻译:

追逐瀑布:烯酰还原酶 (FabI) 与抑制剂复合可稳定四聚体结构并打开水通道
烯酰-ACP 还原酶 (FabI) 是细菌膜脂肪酸生物合成的限制步骤,也是新型抗菌剂的药物靶点。FabI 活性形式是同源四聚体,对抑制剂具有最高的亲和力。在此,使用与已知抑制剂复合的 FabI 结构进行分子动力学研究,以研究它们对四聚化的影响。我们的结果表明,多聚化对于催化位点的完整性至关重要,并且抑制剂结合通过稳定底物结合环(SBL,L:195-200)以及 H4/5(QR 界面)的变化来实现多聚化。我们还观察到 AFN-1252(萘吡啶酮衍生物)促进影响单体-单体界面的独特构象变化。这些变化是由 AFN-1252 与结合位点(Ala95、Tyr146 和 Tyr156)中的关键残基相互作用引起的。此外,水轨迹分析表明,AFN-1252 复合物比三氯生和 MUT056399 复合物允许更多的水分子进入结合位点。FabI–AFN-1252 模拟显示水分子在 Tyr146/147 口袋附近积累,这可能成为新型 FabI 抑制剂设计的热点。
更新日期:2022-11-07
中文翻译:

追逐瀑布:烯酰还原酶 (FabI) 与抑制剂复合可稳定四聚体结构并打开水通道
烯酰-ACP 还原酶 (FabI) 是细菌膜脂肪酸生物合成的限制步骤,也是新型抗菌剂的药物靶点。FabI 活性形式是同源四聚体,对抑制剂具有最高的亲和力。在此,使用与已知抑制剂复合的 FabI 结构进行分子动力学研究,以研究它们对四聚化的影响。我们的结果表明,多聚化对于催化位点的完整性至关重要,并且抑制剂结合通过稳定底物结合环(SBL,L:195-200)以及 H4/5(QR 界面)的变化来实现多聚化。我们还观察到 AFN-1252(萘吡啶酮衍生物)促进影响单体-单体界面的独特构象变化。这些变化是由 AFN-1252 与结合位点(Ala95、Tyr146 和 Tyr156)中的关键残基相互作用引起的。此外,水轨迹分析表明,AFN-1252 复合物比三氯生和 MUT056399 复合物允许更多的水分子进入结合位点。FabI–AFN-1252 模拟显示水分子在 Tyr146/147 口袋附近积累,这可能成为新型 FabI 抑制剂设计的热点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号