Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2022-11-06 , DOI: 10.1016/j.csbj.2022.10.042 David Kim 1 , John Tracey 1 , Manuel Becerra Flores 2 , Kanita Chaudhry 1 , Rafae Nasim 1 , Abraham Correa-Medina 1 , Leslie Knipling 1 , Qing Chen 3 , Scott Stibitz 3 , Lisa M M Jenkins 4 , Kyung Moon 1 , Tim Cardozo 2 , Deborah M Hinton 1
|
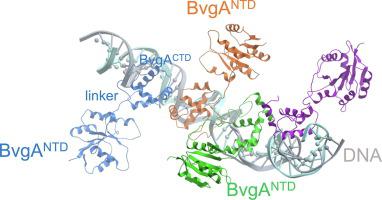
The BvgAS two-component system regulates virulence gene expression in Bordetella pertussis. Although precise three-dimensional structural information is not available for the response regulator BvgA, its sequence conservation with E. coli NarL and previous studies have indicated that it is composed of 3 domains: an N-terminal domain (NTD) containing the phosphorylation site, a linker, and a DNA-binding C-terminal domain (CTD). Previous work has determined how BvgACTD dimers interact with the promoter (PfhaB) of fhaB, the gene encoding the virulence adhesin filamentous hemagglutinin. Here we use molecular modeling, FeBABE footprinting, and crosslinking to show that within the transcription complex of phosphorylated BvgA (BvgA∼P), B. pertussis RNAP, and PfhaB, the NTDs displace from the CTDs and are positioned at specific locations relative to the three BvgA∼P binding sites. Our work identifies a patch of the NTD that faces the DNA and suggests that BvgA∼P undergoes a conformational rearrangement that relocates the NTD to allow productive interaction of the CTD with the DNA.
中文翻译:

博德特氏菌反应调节因子 BvgA 的构象变化伴随着其对百日咳杆菌毒力基因 fhaB 的激活
BvgAS 双组分系统调节百日咳博德特氏菌的毒力基因表达。尽管响应调节因子 BvgA 无法获得精确的三维结构信息,但其与大肠杆菌NarL 的序列保守性和先前的研究表明它由 3 个结构域组成:包含磷酸化位点的 N 末端结构域 (NTD),一个接头和一个 DNA 结合的 C 末端结构域 (CTD)。以前的工作已经确定了 BvgA CTD二聚体如何与 fhaB 的启动子 (P fhaB )相互作用,编码毒力粘附素丝状血凝素的基因。在这里,我们使用分子建模、FeBABE 足迹和交联来显示在磷酸化 BvgA (BvgA∼P)、B. pertussis RNAP 和 P fhaB的转录复合物中, NTD 从 CTD 取代并定位在相对于三个 BvgA∼P 结合位点。我们的工作确定了一块面向 DNA 的 NTD,并表明 BvgA∼P 经历了构象重排,重新定位 NTD 以允许 CTD 与 DNA 进行有效的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号