当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A 1,2,3-Triazole Derivative of Quinazoline Exhibits Antitumor Activity by Tethering RNF168 to SQSTM1/P62
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-11-04 , DOI: 10.1021/acs.jmedchem.2c00432 Fu-Cheng Wang 1, 2 , Bin Peng 1 , Ting-Ting Ren 3 , Shao-Peng Liu 3 , Jing-Rui Du 3 , Zi-Hao Chen 3 , Ting-Ting Zhang 3 , Xiaoyang Gu 4 , Mo Li 4 , Sheng-Li Cao 3 , Xingzhi Xu 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-11-04 , DOI: 10.1021/acs.jmedchem.2c00432 Fu-Cheng Wang 1, 2 , Bin Peng 1 , Ting-Ting Ren 3 , Shao-Peng Liu 3 , Jing-Rui Du 3 , Zi-Hao Chen 3 , Ting-Ting Zhang 3 , Xiaoyang Gu 4 , Mo Li 4 , Sheng-Li Cao 3 , Xingzhi Xu 1
Affiliation
|
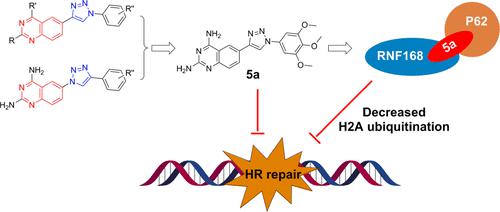
Quinazoline and its derivatives have drawn much attention in the development of potential antitumor agents. Here, we synthesized a series of 1,2,3-triazole derivatives of quinazoline at the C6 position and evaluated for their cytotoxic activity in various human cancer cell lines. We found that compound 5a was the most cytotoxic to HCT-116 cells (IC50, 0.36 μM). Target profiling found that 5a directly binds to both the autophagy-associated protein SQSTM1/P62 and the E3 ligase RNF168, promoting their interaction. Consistently, 5a treatment induces a decrease in RNF168-mediated H2A ubiquitination and compromises homologous recombination-mediated DNA repair, thus increasing the sensitivity of HCT-116 to X-ray radiation. Moreover, 5a suppressed xenografted tumor growth in mice in a dose-dependent manner. Taken together, the 1,2,3-triazole derivative of quinazoline 5a may serve as a novel compound for tumor therapy based on its role in promoting a P62/RNF168 interaction.
中文翻译:

喹唑啉的 1,2,3-三唑衍生物通过将 RNF168 拴系到 SQSTM1/P62 表现出抗肿瘤活性
喹唑啉及其衍生物在潜在抗肿瘤药物的开发中备受关注。在这里,我们在 C6 位合成了一系列喹唑啉的 1,2,3-三唑衍生物,并评估了它们在各种人类癌细胞系中的细胞毒活性。我们发现化合物5a对 HCT-116 细胞的细胞毒性最强(IC 50 , 0.36 μM)。目标分析发现5a直接与自噬相关蛋白 SQSTM1/P62 和 E3 连接酶 RNF168 结合,促进它们的相互作用。一致地,5a处理诱导 RNF168 介导的 H2A 泛素化减少并损害同源重组介导的 DNA 修复,从而增加 HCT-116 对 X 射线辐射的敏感性。而且,5a以剂量依赖性方式抑制小鼠异种移植肿瘤的生长。总之,基于其促进 P62/RNF168 相互作用的作用,喹唑啉5a的 1,2,3-三唑衍生物可用作肿瘤治疗的新型化合物。
更新日期:2022-11-04
中文翻译:

喹唑啉的 1,2,3-三唑衍生物通过将 RNF168 拴系到 SQSTM1/P62 表现出抗肿瘤活性
喹唑啉及其衍生物在潜在抗肿瘤药物的开发中备受关注。在这里,我们在 C6 位合成了一系列喹唑啉的 1,2,3-三唑衍生物,并评估了它们在各种人类癌细胞系中的细胞毒活性。我们发现化合物5a对 HCT-116 细胞的细胞毒性最强(IC 50 , 0.36 μM)。目标分析发现5a直接与自噬相关蛋白 SQSTM1/P62 和 E3 连接酶 RNF168 结合,促进它们的相互作用。一致地,5a处理诱导 RNF168 介导的 H2A 泛素化减少并损害同源重组介导的 DNA 修复,从而增加 HCT-116 对 X 射线辐射的敏感性。而且,5a以剂量依赖性方式抑制小鼠异种移植肿瘤的生长。总之,基于其促进 P62/RNF168 相互作用的作用,喹唑啉5a的 1,2,3-三唑衍生物可用作肿瘤治疗的新型化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号