Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Testing of drugs using human feto-maternal interface organ-on-chips provide insights into pharmacokinetics and efficacy
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-11-02 , DOI: 10.1039/d2lc00691j Lauren S Richardson 1 , Ananth K Kammala 1 , Maged M Costantine 2 , Stephen J Fortunato 3 , Enkhtuya Radnaa 1 , Sungjin Kim 4 , Robert N Taylor 5 , Arum Han 4 , Ramkumar Menon 1
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-11-02 , DOI: 10.1039/d2lc00691j Lauren S Richardson 1 , Ananth K Kammala 1 , Maged M Costantine 2 , Stephen J Fortunato 3 , Enkhtuya Radnaa 1 , Sungjin Kim 4 , Robert N Taylor 5 , Arum Han 4 , Ramkumar Menon 1
Affiliation
|
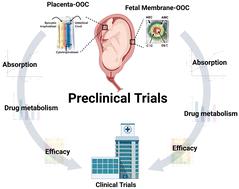
Objectives: To improve preclinical drug testing during pregnancy, we developed multiple microfluidic organ-on-chip (OOC) devices that represent the structure, functions, and responses of the two feto-maternal interfaces (FMis) in humans (fetal membrane [FMi-OOC] and placenta [PLA-OOC]). This study utilized feto-maternal interface OOCs to test the kinetics and efficacy of drugs during pregnancy. Study design: The FMi-OOC contained amnion epithelial, mesenchymal, chorion trophoblast, and decidual cells. The PLA-OOC contained cytotrophoblasts (BeWo), syncytiotrophoblasts (BeWo + forskolin), and human umbilical vein endothelial cell lines. Therapeutic concentrations of either pravastatin or rosuvastatin (200 ng mL−1), a model drug for these experiments, were applied to either decidua (in FMi-OOC) and syncytiotrophoblasts (in PLA-OOC) chambers under normal and oxidative stress conditions (induced by cigarette smoke extract [CSE 1 : 25]) to evaluate maternal drug exposure during normal pregnancy or oxidative stress (OS) associated pathologies, respectively. We determined statin pharmacokinetics and metabolism (LC-MS/MS), drug-induced cytotoxicity (LDH assay), and efficacy to reduce OS-induced inflammation (multiplex cytokine assay). Results: Both OOCs mimicked two distinct human feto-maternal interfaces. The drugs tested permeated the maternal–fetal cell layers of the FMi-OOC and PLA-OOC within 4 hours and generated cell and time-specific statin metabolites from various cell types without causing any cytotoxicity. OS-induced pro-inflammatory cytokines were effectively reduced by statins by increasing anti-inflammatory cytokine response across the FMi-OOC and PLA-OOC. Conclusion: Two distinct feto-maternal interface OOCs were developed, tested, and validated for their utility to conduct preclinical trials during pregnancy. We demonstrated that the placenta and fetal membranes-decidual interface both are able to transport and metabolize drugs and that the safety and efficacy of a drug can be determined using the anatomical structures recreated on OOCs.
中文翻译:

使用人类胎儿-母体界面器官芯片测试药物提供了对药代动力学和功效的深入了解
目的:为了改进怀孕期间的临床前药物测试,我们开发了多个微流体片上器官 (OOC) 装置,这些装置代表了人类两个胎儿-母体界面 (FMis) 的结构、功能和反应(胎膜 [FMi- OOC] 和胎盘 [PLA-OOC])。本研究利用胎儿-母体界面 OOC 来测试药物在妊娠期间的动力学和功效。研究设计:FMi-OOC 包含羊膜上皮细胞、间充质细胞、绒毛膜滋养细胞和蜕膜细胞。PLA-OOC 包含细胞滋养层细胞 (BeWo)、合体滋养层细胞 (BeWo + forskolin) 和人脐静脉内皮细胞系。普伐他汀或瑞舒伐他汀的治疗浓度(200 ng mL -1),一种用于这些实验的模型药物,在正常和氧化应激条件下(由香烟烟雾提取物 [CSE 1 : 25] 诱导)应用于蜕膜(在 FMi-OOC 中)和合体滋养层细胞(在 PLA-OOC 中)以评估分别在正常妊娠或氧化应激 (OS) 相关病理期间母体药物暴露。我们确定了他汀类药物的药代动力学和代谢 (LC-MS/MS)、药物诱导的细胞毒性(LDH 测定)和减少 OS 诱导的炎症的功效(多重细胞因子测定)。结果:两个 OOC 都模仿了两个截然不同的人类胎儿-母体界面。测试的药物在 4 小时内渗透到 FMi-OOC 和 PLA-OOC 的母胎细胞层,并从各种细胞类型中产生细胞和时间特异性他汀类药物代谢物,而不会引起任何细胞毒性。通过增加 FMi-OOC 和 PLA-OOC 的抗炎细胞因子反应,他汀类药物有效减少了 OS 诱导的促炎细胞因子。结论:开发、测试和验证了两种不同的胎儿-母体界面 OOC,以证明它们在怀孕期间进行临床前试验的效用。我们证明了胎盘和胎膜-蜕膜界面都能够运输和代谢药物,并且可以使用在 OOCs 上重建的解剖结构来确定药物的安全性和有效性。
更新日期:2022-11-02
中文翻译:

使用人类胎儿-母体界面器官芯片测试药物提供了对药代动力学和功效的深入了解
目的:为了改进怀孕期间的临床前药物测试,我们开发了多个微流体片上器官 (OOC) 装置,这些装置代表了人类两个胎儿-母体界面 (FMis) 的结构、功能和反应(胎膜 [FMi- OOC] 和胎盘 [PLA-OOC])。本研究利用胎儿-母体界面 OOC 来测试药物在妊娠期间的动力学和功效。研究设计:FMi-OOC 包含羊膜上皮细胞、间充质细胞、绒毛膜滋养细胞和蜕膜细胞。PLA-OOC 包含细胞滋养层细胞 (BeWo)、合体滋养层细胞 (BeWo + forskolin) 和人脐静脉内皮细胞系。普伐他汀或瑞舒伐他汀的治疗浓度(200 ng mL -1),一种用于这些实验的模型药物,在正常和氧化应激条件下(由香烟烟雾提取物 [CSE 1 : 25] 诱导)应用于蜕膜(在 FMi-OOC 中)和合体滋养层细胞(在 PLA-OOC 中)以评估分别在正常妊娠或氧化应激 (OS) 相关病理期间母体药物暴露。我们确定了他汀类药物的药代动力学和代谢 (LC-MS/MS)、药物诱导的细胞毒性(LDH 测定)和减少 OS 诱导的炎症的功效(多重细胞因子测定)。结果:两个 OOC 都模仿了两个截然不同的人类胎儿-母体界面。测试的药物在 4 小时内渗透到 FMi-OOC 和 PLA-OOC 的母胎细胞层,并从各种细胞类型中产生细胞和时间特异性他汀类药物代谢物,而不会引起任何细胞毒性。通过增加 FMi-OOC 和 PLA-OOC 的抗炎细胞因子反应,他汀类药物有效减少了 OS 诱导的促炎细胞因子。结论:开发、测试和验证了两种不同的胎儿-母体界面 OOC,以证明它们在怀孕期间进行临床前试验的效用。我们证明了胎盘和胎膜-蜕膜界面都能够运输和代谢药物,并且可以使用在 OOCs 上重建的解剖结构来确定药物的安全性和有效性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号