当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From Peripheral Stereogenic Center to Axial Chirality: Synthesis of 3-Arylthiophene Atropisomers
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-01 , DOI: 10.1002/adsc.202200957 Lin Li 1 , Junwei Xi 1 , Biqiong Hong 2 , Zhenhua Gu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-01 , DOI: 10.1002/adsc.202200957 Lin Li 1 , Junwei Xi 1 , Biqiong Hong 2 , Zhenhua Gu 1
Affiliation
|
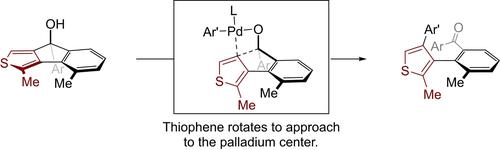
A practical synthesis of axially chiral biaryls via chirality transfer from peripheral stereogenic carbon centers is reported. In contrast to the traditional aromatization process of a non-aromatic structure with ipso stereogenic centers, this method is based on a Pd-catalyzed carbon-carbon bond cleavage/ring-opening reaction of optically active 8H-indenothiophen-8-ols with an achiral ligand, enabling synthesis of highly functionalized 3-arylthiophene atropisomers in high enantioselectivity.
中文翻译:

从外围立体中心到轴向手性:3-芳基噻吩阻转异构体的合成
报道了通过从外围立体异构碳中心进行的手性转移来实际合成轴向手性联芳基化合物。与具有ipso手性中心的非芳香结构的传统芳构化过程相比,该方法基于 Pd 催化的具有光学活性的 8 H -茚并噻吩 -8-醇的碳 - 碳键裂解/开环反应非手性配体,能够以高对映选择性合成高度功能化的 3-芳基噻吩阻转异构体。
更新日期:2022-11-01
中文翻译:

从外围立体中心到轴向手性:3-芳基噻吩阻转异构体的合成
报道了通过从外围立体异构碳中心进行的手性转移来实际合成轴向手性联芳基化合物。与具有ipso手性中心的非芳香结构的传统芳构化过程相比,该方法基于 Pd 催化的具有光学活性的 8 H -茚并噻吩 -8-醇的碳 - 碳键裂解/开环反应非手性配体,能够以高对映选择性合成高度功能化的 3-芳基噻吩阻转异构体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号