Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards personalized antibody cancer therapy: development of a microfluidic cell culture device for antibody selection
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-11-02 , DOI: 10.1039/d2lc00918h Pedro G M Condelipes 1, 2 , Pedro Mendes Fontes 1, 3 , Ana Godinho-Santos 3 , Eduardo J S Brás 1, 4 , Vanda Marques 3 , Marta B Afonso 3 , Cecília M P Rodrigues 3 , Virginia Chu 1 , João Gonçalves 3 , João Pedro Conde 1, 2
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-11-02 , DOI: 10.1039/d2lc00918h Pedro G M Condelipes 1, 2 , Pedro Mendes Fontes 1, 3 , Ana Godinho-Santos 3 , Eduardo J S Brás 1, 4 , Vanda Marques 3 , Marta B Afonso 3 , Cecília M P Rodrigues 3 , Virginia Chu 1 , João Gonçalves 3 , João Pedro Conde 1, 2
Affiliation
|
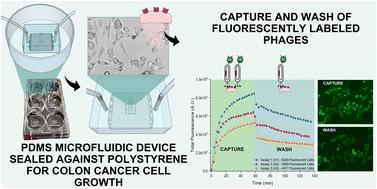
Antibody therapy has been one of the most successful therapies for a wide range of diseases, including cancer. One way of expediting antibody therapy development is through phage display technology. Here, by screening thousands of randomly assembled peptide sequences, it is possible to identify potential therapeutic candidates. Conventional screening technologies do not accommodate perfusion through the system, as is the case of standard plate-based cultures. This leads to a poor translation of the experimental results obtained in vitro when moving to a more physiologically relevant setting, such as the case of preclinical animal models or clinical trials. Microfluidics is a technology that can improve screening efficacy by replicating more physiologically relevant conditions such as shear stress. In this work, a polydimethylsiloxane/polystyrene-based microfluidic system for a continuously perfused culture of cancer cells is reported. Human colorectal adenocarcinoma cells (HCT116) expressing CXCR4 were used as a cell target. Fluorescently labeled M13 phages anti-CXCR4 were used to study the efficiency of the microfluidic system as a tool to study the binding kinetics of the engineered bacteriophages. Using our microfluidic platform, we estimated a dissociation constant of 0.45 pM for the engineered phage. Additionally, a receptor internalization assay was developed using SDF-1α to verify phage specificity to the CXCR4 receptor. Upon receptor internalization there was a signal reduction, proving that the anti-CXCR4 fluorescently labelled M13 phages bound specifically to the CXCR4 receptor. The simplicity and ease of use of the microfluidic device design presented in this work can form the basis of a generic platform that facilitates the study and optimization of therapies based on interaction with biological entities such as mammalian cells.
中文翻译:

迈向个性化抗体癌症治疗:开发用于抗体选择的微流控细胞培养装置
抗体疗法一直是包括癌症在内的多种疾病最成功的疗法之一。加快抗体疗法开发的一种方法是通过噬菌体展示技术。在这里,通过筛选数千个随机组装的肽序列,可以识别潜在的治疗候选物。传统的筛选技术无法通过系统进行灌注,标准的基于平板的培养就是这种情况。这导致体外获得的实验结果翻译不佳当转移到更生理相关的环境时,例如临床前动物模型或临床试验的情况。微流体是一种可以通过复制更多生理相关条件(例如剪切应力)来提高筛选效率的技术。在这项工作中,报道了一种用于连续灌注癌细胞培养的基于聚二甲基硅氧烷/聚苯乙烯的微流体系统。表达 CXCR4 的人结直肠腺癌细胞 (HCT116) 用作细胞靶标。荧光标记的 M13 噬菌体抗 CXCR4 用于研究微流体系统的效率,作为研究工程噬菌体结合动力学的工具。使用我们的微流体平台,我们估计工程噬菌体的解离常数为 0.45 pM。此外,使用 SDF-1α 开发了受体内化试验,以验证噬菌体对 CXCR4 受体的特异性。受体内化后信号减少,证明抗 CXCR4 荧光标记的 M13 噬菌体特异性结合 CXCR4 受体。这项工作中介绍的微流体设备设计的简单性和易用性可以构成一个通用平台的基础,该平台可以促进基于与哺乳动物细胞等生物实体相互作用的疗法的研究和优化。
更新日期:2022-11-02
中文翻译:

迈向个性化抗体癌症治疗:开发用于抗体选择的微流控细胞培养装置
抗体疗法一直是包括癌症在内的多种疾病最成功的疗法之一。加快抗体疗法开发的一种方法是通过噬菌体展示技术。在这里,通过筛选数千个随机组装的肽序列,可以识别潜在的治疗候选物。传统的筛选技术无法通过系统进行灌注,标准的基于平板的培养就是这种情况。这导致体外获得的实验结果翻译不佳当转移到更生理相关的环境时,例如临床前动物模型或临床试验的情况。微流体是一种可以通过复制更多生理相关条件(例如剪切应力)来提高筛选效率的技术。在这项工作中,报道了一种用于连续灌注癌细胞培养的基于聚二甲基硅氧烷/聚苯乙烯的微流体系统。表达 CXCR4 的人结直肠腺癌细胞 (HCT116) 用作细胞靶标。荧光标记的 M13 噬菌体抗 CXCR4 用于研究微流体系统的效率,作为研究工程噬菌体结合动力学的工具。使用我们的微流体平台,我们估计工程噬菌体的解离常数为 0.45 pM。此外,使用 SDF-1α 开发了受体内化试验,以验证噬菌体对 CXCR4 受体的特异性。受体内化后信号减少,证明抗 CXCR4 荧光标记的 M13 噬菌体特异性结合 CXCR4 受体。这项工作中介绍的微流体设备设计的简单性和易用性可以构成一个通用平台的基础,该平台可以促进基于与哺乳动物细胞等生物实体相互作用的疗法的研究和优化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号