当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strategies toward the Difunctionalizations of Enamide Derivatives for Synthesizing α,β-Substituted Amines
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-11-01 , DOI: 10.1021/acs.accounts.2c00540 Damien Bouchet 1 , Thomas Varlet 1 , Géraldine Masson 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-11-01 , DOI: 10.1021/acs.accounts.2c00540 Damien Bouchet 1 , Thomas Varlet 1 , Géraldine Masson 1, 2
Affiliation
|
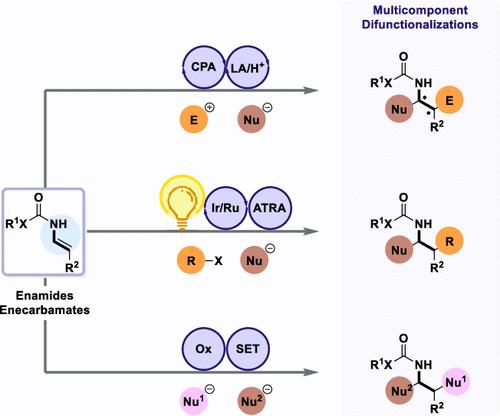
Enamide and enecarbamate derivatives containing a nucleophilic center at the β-position from their nitrogen atom as well as a latent electrophilic site at their α-position are interesting motifs in organic chemistry. This dual reactivity─analogous that of the enamines─enables difunctionalization and increased structural complexity. Furthermore, an electron-withdrawing group on nitrogen drastically increases their stability. In that respect, enamides and enecarbamates are excellent partners for multicomponent transformations, and our research primarily focuses on these compounds in particular.
中文翻译:

用于合成 α,β-取代胺的烯酰胺衍生物双官能化策略
烯酰胺和烯氨基甲酸酯衍生物在其氮原子的 β 位含有一个亲核中心,在其 α 位含有一个潜在的亲电位点,是有机化学中有趣的基序。这种双重反应性——类似于烯胺——使双功能化和结构复杂性增加成为可能。此外,氮上的吸电子基团大大增加了它们的稳定性。在这方面,烯酰胺和烯氨基甲酸酯是多组分转化的优秀合作伙伴,我们的研究主要集中在这些化合物上。
更新日期:2022-11-01
中文翻译:

用于合成 α,β-取代胺的烯酰胺衍生物双官能化策略
烯酰胺和烯氨基甲酸酯衍生物在其氮原子的 β 位含有一个亲核中心,在其 α 位含有一个潜在的亲电位点,是有机化学中有趣的基序。这种双重反应性——类似于烯胺——使双功能化和结构复杂性增加成为可能。此外,氮上的吸电子基团大大增加了它们的稳定性。在这方面,烯酰胺和烯氨基甲酸酯是多组分转化的优秀合作伙伴,我们的研究主要集中在这些化合物上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号