当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemically Induced Regio- and Stereoselective (E)-β-C(sp2)−H Trifluoromethylation and Arylsulfonylation of Enamides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-01 , DOI: 10.1002/adsc.202200934 Fukuan Zhang 1 , Xuefei Zhao 2 , Jie Zhang 1 , Lili Zhao 2 , Lin Li 1 , Jiayi Yang 1 , Hao Li 1 , Haiqing Luo 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-01 , DOI: 10.1002/adsc.202200934 Fukuan Zhang 1 , Xuefei Zhao 2 , Jie Zhang 1 , Lili Zhao 2 , Lin Li 1 , Jiayi Yang 1 , Hao Li 1 , Haiqing Luo 1
Affiliation
|
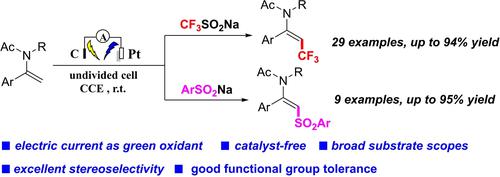
Herein, we disclosed an electrochemically induced method for the regio- and stereoselective (E)-β-C(sp2)−H trifluoromethylation of enamides by employing readily available and inexpensive Langlois’ reagent (CF3SO2Na). Preliminary mechanistic studies indicate the involvement of free radicals in the process. The exogenous oxidant-free reaction proceeds in an undivided electrochemical cell under mild conditions and allows for the accomplishment of the trifluoromethylation products with exclusive E-selective control. The methodology is featured by catalyst-free, simple setup, and broad substrate scopes of enamides with good functional group tolerance. Using ArSO2Na as the coupling partner, the corresponding (E)-β-C(sp2)−H arylsulfonylated enamides products are obtained under standard reaction conditions.
中文翻译:

电化学诱导的区域选择性和立体选择性 (E)-β-C(sp2)−H 三氟甲基化和烯酰胺的芳基磺酰化
在此,我们公开了一种电化学诱导的区域选择性和立体选择性 ( E )-β-C( sp 2 )-H 三氟甲基化的方法,使用现成且廉价的 Langlois 试剂 (CF 3 SO 2 Na)。初步的机理研究表明自由基参与了这个过程。无外源氧化剂的反应在温和条件下在未分隔的电化学电池中进行,并允许通过独家E选择性控制完成三氟甲基化产物。该方法的特点是无催化剂、设置简单、烯酰胺底物范围广、官能团耐受性好。使用 ArSO 2Na 作为偶联配偶体,在标准反应条件下得到相应的 ( E )-β-C( sp 2 )−H 芳基磺酰化烯酰胺产物。
更新日期:2022-11-01
中文翻译:

电化学诱导的区域选择性和立体选择性 (E)-β-C(sp2)−H 三氟甲基化和烯酰胺的芳基磺酰化
在此,我们公开了一种电化学诱导的区域选择性和立体选择性 ( E )-β-C( sp 2 )-H 三氟甲基化的方法,使用现成且廉价的 Langlois 试剂 (CF 3 SO 2 Na)。初步的机理研究表明自由基参与了这个过程。无外源氧化剂的反应在温和条件下在未分隔的电化学电池中进行,并允许通过独家E选择性控制完成三氟甲基化产物。该方法的特点是无催化剂、设置简单、烯酰胺底物范围广、官能团耐受性好。使用 ArSO 2Na 作为偶联配偶体,在标准反应条件下得到相应的 ( E )-β-C( sp 2 )−H 芳基磺酰化烯酰胺产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号