当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel, Practical, and Efficient Process for the Preparation of 4,5-Dichloroindole
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2022-10-31 , DOI: 10.1021/acs.oprd.2c00212 Prajna Parimita Mohanta 1 , Ajaya Kumar Behera 1 , Hari Narayan Pati 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2022-10-31 , DOI: 10.1021/acs.oprd.2c00212 Prajna Parimita Mohanta 1 , Ajaya Kumar Behera 1 , Hari Narayan Pati 1
Affiliation
|
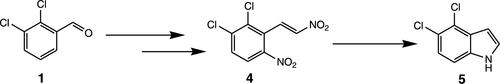
A novel, practical, and efficient three-step process for the preparation of 4,5-dichloroindole 5, an important starting material for a wide range of fine chemicals and pharmaceuticals has been developed. The process comprises nitration of commercially available 2,3-dichlorobenzaldehyde 1, a telescopic process for the Henry reaction, and subsequently reductive cyclization of resulting o,β-dinitrostyrene intermediate 4 into 4,5-dichloroindole using iron powder in methanol and acetic acid by the Nenitzescu reaction. The large-scale applicability of this novel and improved process has been successfully demonstrated on a multikilogram scale by carrying multiple batches to produce 5 in 67–70% yields and 96–98% purity without column chromatography. The reactions are facile, safe, and easy to scale up.
中文翻译:

新型、实用、高效的 4,5-二氯吲哚制备工艺
已开发出一种新颖、实用且高效的三步法制备 4,5-二氯吲哚5,这是多种精细化学品和药物的重要起始原料。该工艺包括市售 2,3-二氯苯甲醛1的硝化,亨利反应的伸缩过程,以及随后使用铁粉在甲醇和乙酸中通过以下方法将所得o ,β-二硝基苯乙烯中间体4还原环化为 4,5-二氯吲哚尼尼采斯库反应。这种新颖和改进的工艺的大规模适用性已通过多批生产5无需柱层析,产率为 67–70%,纯度为 96–98%。反应简单、安全且易于放大。
更新日期:2022-10-31
中文翻译:

新型、实用、高效的 4,5-二氯吲哚制备工艺
已开发出一种新颖、实用且高效的三步法制备 4,5-二氯吲哚5,这是多种精细化学品和药物的重要起始原料。该工艺包括市售 2,3-二氯苯甲醛1的硝化,亨利反应的伸缩过程,以及随后使用铁粉在甲醇和乙酸中通过以下方法将所得o ,β-二硝基苯乙烯中间体4还原环化为 4,5-二氯吲哚尼尼采斯库反应。这种新颖和改进的工艺的大规模适用性已通过多批生产5无需柱层析,产率为 67–70%,纯度为 96–98%。反应简单、安全且易于放大。
































 京公网安备 11010802027423号
京公网安备 11010802027423号