当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational design of arsine catalysts for arsa-Wittig reaction
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-10-27 , DOI: 10.1039/d2qo01480g Junya Yukiyasu 1 , Ryoto Inaba 1 , Takashi Yumura 2 , Hiroaki Imoto 1 , Kensuke Naka 1, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-10-27 , DOI: 10.1039/d2qo01480g Junya Yukiyasu 1 , Ryoto Inaba 1 , Takashi Yumura 2 , Hiroaki Imoto 1 , Kensuke Naka 1, 3
Affiliation
|
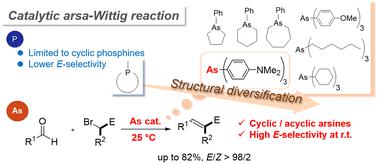
The catalytic Wittig reaction is widely used for obtaining alkenes; it avoids the generation of stoichiometric amounts of phosphine oxide. The catalytic arsa-Wittig reaction, in which an arsine catalyst is used instead of phosphine, has the advantage of excellent E-selectivity. However, in our recent study, we reported that high temperatures are required for the reaction to proceed due to the low nucleophilicity in arsines. In this study, we prepared cyclic and acyclic arsines with electron-donating and/or sterically open substituents and examined their catalytic activities. The results showed that tris(p-(dimethylamino)phenyl)arsine (5) exhibited high catalytic activity despite being an acyclic arsine; the traditional phosphine-catalyzed Wittig reaction requires a cyclic phosphine catalyst. Under the optimized reaction conditions, the room-temperature arsa-Wittig reaction proceeded with high yield (up to 82%) and excellent E-selectivity (>98%). Further, using 5 as a catalyst, density functional theory calculations were performed to elucidate the mechanism of the arsa-Wittig reaction.
中文翻译:

用于arsa-Wittig反应的胂催化剂的合理设计
催化 Wittig 反应广泛用于获得烯烃;它避免了化学计量的氧化膦的产生。使用胂催化剂代替膦的催化arsa-Wittig反应具有优异的E-选择性优势。然而,在我们最近的研究中,我们报道了由于胂的低亲核性,反应需要高温才能进行。在这项研究中,我们制备了具有给电子和/或空间开放取代基的环状和无环胂,并检查了它们的催化活性。结果表明,三(对-(二甲氨基)苯基)胂(5) 尽管是无环胂,但表现出高催化活性;传统的膦催化 Wittig 反应需要环状膦催化剂。在优化的反应条件下,室温 arsa-Wittig 反应以高产率(高达 82%)和优异的E选择性(>98%)进行。此外,使用5作为催化剂,进行了密度泛函理论计算,以阐明 arsa-Wittig 反应的机理。
更新日期:2022-10-31
中文翻译:

用于arsa-Wittig反应的胂催化剂的合理设计
催化 Wittig 反应广泛用于获得烯烃;它避免了化学计量的氧化膦的产生。使用胂催化剂代替膦的催化arsa-Wittig反应具有优异的E-选择性优势。然而,在我们最近的研究中,我们报道了由于胂的低亲核性,反应需要高温才能进行。在这项研究中,我们制备了具有给电子和/或空间开放取代基的环状和无环胂,并检查了它们的催化活性。结果表明,三(对-(二甲氨基)苯基)胂(5) 尽管是无环胂,但表现出高催化活性;传统的膦催化 Wittig 反应需要环状膦催化剂。在优化的反应条件下,室温 arsa-Wittig 反应以高产率(高达 82%)和优异的E选择性(>98%)进行。此外,使用5作为催化剂,进行了密度泛函理论计算,以阐明 arsa-Wittig 反应的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号