当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Supramolecular Phthalimide Networks Via Tandem Diels–Alder Reaction–Aromatization Using Biomass-Derived Furanic Dienes
Macromolecular Rapid Communications ( IF 4.6 ) Pub Date : 2022-10-25 , DOI: 10.1002/marc.202200711 Byounghyun Kim 1 , Juhyen Lee 1 , Han Yong Bae 1 , Seung Uk Son 1 , Changsik Song 1
Macromolecular Rapid Communications ( IF 4.6 ) Pub Date : 2022-10-25 , DOI: 10.1002/marc.202200711 Byounghyun Kim 1 , Juhyen Lee 1 , Han Yong Bae 1 , Seung Uk Son 1 , Changsik Song 1
Affiliation
|
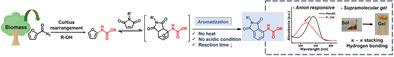
The design and synthesis of phthalimide derivatives are important goals for applications in fields such as pharmaceutical science and optoelectronics. In the present study, a facile and convenient synthetic pathway (no heat or acid/catalyst needed) is devised to produce phthalimides from a biomass-derived furan by directly introducing an N-carbamate group at the C-2 position of the furan ring via thermal Curtius rearrangement. The electron-donating N-carbamate group increases the energy level of the highest occupied molecular orbital of the furan diene, resulting in a significant increase of the rate of the Diels–Alder reaction with maleimide compared to the conventional furfuryl furan. Interestingly, the Diels–Alder adduct smoothly undergoes aromatization (dehydration) to generate the phthalimide motif. It is shown that the biomass-derived phthalimides can produce supramolecular gels and act as sensors of basic anions like F− and CN−. The novel synthetic pathway to phthalimide derivatives from a biomass-derived furan can potentially be used to develop novel phthalimide motifs for a variety of applications.
中文翻译:

超分子邻苯二甲酰亚胺网络通过串联 Diels-Alder 反应-使用生物质衍生的呋喃二烯芳构化
邻苯二甲酰亚胺衍生物的设计和合成是药物科学和光电子等领域应用的重要目标。在本研究中,设计了一种简便的合成途径(无需加热或酸/催化剂),通过在呋喃环的 C-2 位直接引入N-氨基甲酸酯基团,从生物质衍生的呋喃生产邻苯二甲酰亚胺热 Curtius 重排。给电子N-氨基甲酸酯基团增加了呋喃二烯最高占据分子轨道的能级,导致与传统糠基呋喃相比,马来酰亚胺的 Diels-Alder 反应速率显着增加。有趣的是,Diels-Alder 加合物顺利进行芳构化(脱水)以生成邻苯二甲酰亚胺基序。结果表明,生物质衍生的邻苯二甲酰亚胺可以产生超分子凝胶,并充当 F -和 CN -等碱性阴离子的传感器。从生物质衍生的呋喃到邻苯二甲酰亚胺衍生物的新合成途径可潜在地用于开发用于各种应用的新邻苯二甲酰亚胺基序。
更新日期:2022-10-25
中文翻译:

超分子邻苯二甲酰亚胺网络通过串联 Diels-Alder 反应-使用生物质衍生的呋喃二烯芳构化
邻苯二甲酰亚胺衍生物的设计和合成是药物科学和光电子等领域应用的重要目标。在本研究中,设计了一种简便的合成途径(无需加热或酸/催化剂),通过在呋喃环的 C-2 位直接引入N-氨基甲酸酯基团,从生物质衍生的呋喃生产邻苯二甲酰亚胺热 Curtius 重排。给电子N-氨基甲酸酯基团增加了呋喃二烯最高占据分子轨道的能级,导致与传统糠基呋喃相比,马来酰亚胺的 Diels-Alder 反应速率显着增加。有趣的是,Diels-Alder 加合物顺利进行芳构化(脱水)以生成邻苯二甲酰亚胺基序。结果表明,生物质衍生的邻苯二甲酰亚胺可以产生超分子凝胶,并充当 F -和 CN -等碱性阴离子的传感器。从生物质衍生的呋喃到邻苯二甲酰亚胺衍生物的新合成途径可潜在地用于开发用于各种应用的新邻苯二甲酰亚胺基序。



























 京公网安备 11010802027423号
京公网安备 11010802027423号