Journal of Catalysis ( IF 7.3 ) Pub Date : 2022-10-22 , DOI: 10.1016/j.jcat.2022.10.012 Bing Liu , Mengyuan Huang , Zhihao Fang , Lian Kong , Yuebing Xu , Zaijun Li , Xiaohao Liu
|
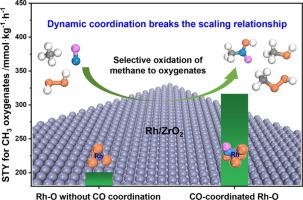
Direct conversion of methane to value-added oxygenate products is of considerable importance for effective valorization of methane, but remains a grand challenge in heterogeneous catalysis due to the high energy barrier required for the first C H bond activation and facile overoxidation of products. Generally, there exists a scaling relationship in direct conversion of methane that is a lower activation energy for methane dissociation always accompanies with undesired lower activation energy for overoxidation. In this study, by combining theoretical calculations and experiments, we systematically investigated the CO-assisted low-temperature selective oxidation of methane using H2O2 under aqueous conditions over atomically dispersed Rh/ZrO2. The results reveal that the introduction of CO on Rh/ZrO2 breaks the scaling relationship, which not only facilitates methane activation and conversion benefiting from the Rh-CO coordination, leading to a substantial enhancement of oxygenate products yield, but also prevents the overoxidation of CH3 species, achieving the improvement of methane activation and suppression of overoxidation concurrently. The dynamic metal-intermediate coordination-induced reactivity modulation mechanism was unveiled, in which electronic state and catalytic property of Rh-O active center dynamically changes along with the change of Rh-intermediate coordination during the reaction, giving rise to the dynamic shift of reactivity descriptors towards more optimal values and consequently enabling the facilitation of the initial C
H bond activation and facile overoxidation of products. Generally, there exists a scaling relationship in direct conversion of methane that is a lower activation energy for methane dissociation always accompanies with undesired lower activation energy for overoxidation. In this study, by combining theoretical calculations and experiments, we systematically investigated the CO-assisted low-temperature selective oxidation of methane using H2O2 under aqueous conditions over atomically dispersed Rh/ZrO2. The results reveal that the introduction of CO on Rh/ZrO2 breaks the scaling relationship, which not only facilitates methane activation and conversion benefiting from the Rh-CO coordination, leading to a substantial enhancement of oxygenate products yield, but also prevents the overoxidation of CH3 species, achieving the improvement of methane activation and suppression of overoxidation concurrently. The dynamic metal-intermediate coordination-induced reactivity modulation mechanism was unveiled, in which electronic state and catalytic property of Rh-O active center dynamically changes along with the change of Rh-intermediate coordination during the reaction, giving rise to the dynamic shift of reactivity descriptors towards more optimal values and consequently enabling the facilitation of the initial C H bond activation while suppression of the following overoxidation. This study opens up new perspectives to tune the catalytic performance and offers a comprehensive picture of the dynamics of atomically dispersed Rh-based catalysts in the field of selective oxidation of methane.
H bond activation while suppression of the following overoxidation. This study opens up new perspectives to tune the catalytic performance and offers a comprehensive picture of the dynamics of atomically dispersed Rh-based catalysts in the field of selective oxidation of methane.
中文翻译:

通过在原子分散的 Rh/ZrO2 催化剂上通过动态金属-中间体配位诱导调节反应性描述符来打破甲烷选择性氧化的比例关系
将甲烷直接转化为高附加值的含氧化合物产品对于甲烷的有效增值具有相当重要的意义,但由于第一个 C  H 键活化和产品的容易过氧化所需的高能垒,在多相催化中仍然是一个巨大的挑战。通常,甲烷的直接转化存在比例关系,即较低的甲烷解离活化能总是伴随着不希望的较低的过氧化活化能。在本研究中,我们通过理论计算和实验相结合,系统地研究了在原子分散的Rh/ZrO 2上在水性条件下使用H 2 O 2的CO 辅助低温选择性氧化甲烷。. 结果表明,在 Rh/ZrO 2上引入 CO打破了比例关系,不仅有利于甲烷的活化和转化,受益于 Rh-CO 配位,从而显着提高了含氧产物的收率,而且还防止了甲烷的过氧化。 CH 3种,同时实现甲烷活化的改善和过氧化的抑制。揭示了动态金属-中间体配位诱导的反应调节机制,其中Rh-O活性中心的电子态和催化性质随着反应过程中Rh-中间体配位的变化而动态变化,从而引起反应性的动态变化描述符趋向于更优化的值,因此能够促进初始 C
H 键活化和产品的容易过氧化所需的高能垒,在多相催化中仍然是一个巨大的挑战。通常,甲烷的直接转化存在比例关系,即较低的甲烷解离活化能总是伴随着不希望的较低的过氧化活化能。在本研究中,我们通过理论计算和实验相结合,系统地研究了在原子分散的Rh/ZrO 2上在水性条件下使用H 2 O 2的CO 辅助低温选择性氧化甲烷。. 结果表明,在 Rh/ZrO 2上引入 CO打破了比例关系,不仅有利于甲烷的活化和转化,受益于 Rh-CO 配位,从而显着提高了含氧产物的收率,而且还防止了甲烷的过氧化。 CH 3种,同时实现甲烷活化的改善和过氧化的抑制。揭示了动态金属-中间体配位诱导的反应调节机制,其中Rh-O活性中心的电子态和催化性质随着反应过程中Rh-中间体配位的变化而动态变化,从而引起反应性的动态变化描述符趋向于更优化的值,因此能够促进初始 C  H 键活化,同时抑制随后的过氧化。这项研究为调整催化性能开辟了新的视角,并全面了解了原子分散的 Rh 基催化剂在甲烷选择性氧化领域的动力学。
H 键活化,同时抑制随后的过氧化。这项研究为调整催化性能开辟了新的视角,并全面了解了原子分散的 Rh 基催化剂在甲烷选择性氧化领域的动力学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号