当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DFT study on ruthenium-catalyzed N-methylbenzamide-directed 1,4-addition of the ortho C–H bond to maleimide via C–H/C–C activation
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-10-22 , DOI: 10.1039/d2qo01487d Zi-Hao Wu 1 , De-Cai Fang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-10-22 , DOI: 10.1039/d2qo01487d Zi-Hao Wu 1 , De-Cai Fang 1
Affiliation
|
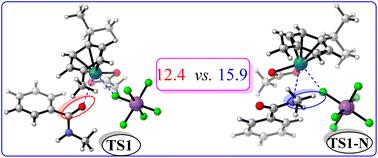
Density functional theory has been used to investigate ruthenium-catalyzed N-methylbenzamide directed 1,4-addition of the ortho C–H bond to maleimide via C–H/C–C activation, in which the active catalyst was proved to be ionic Ru(OAc)(SbF6)(p-pymene) and not neutral Ru(OAc)2(p-pymene). The plausible catalytic cycles involve C–H activation, maleimide insertion and protonation, and the recovery of the catalyst and the formation of the final product. The calculations confirmed that the fragment of SbF6 could leave the ligand field of the Ru center to form N–H⋯F hydrogen bonding when the O atom in the carbonyl group of the first reactant 1a approaches the Ru center, and it could return to the Ru coordination center in the last step to complete the recovery of the catalyst and the formation of the final product. It was deduced from the energy span model that the TOF-determining transition state (TDTS) and intermediate (TDI) are TS4 and INT1, and thus the activation free-energy barrier is 28.2 kcal mol−1 at a temperature of 373 K. The effects of the solvent, the directing group and catalyst type have been addressed in this paper.
中文翻译:

钌催化 N-甲基苯甲酰胺定向 1,4-加成邻位 C-H 键通过 C-H/C-C 活化与马来酰亚胺的 DFT 研究
密度泛函理论已用于研究钌催化的N-甲基苯甲酰胺通过C-H/C-C 活化将邻位C-H 键定向 1,4-加成到马来酰亚胺,其中活性催化剂被证明是离子型 Ru (OAc)(SbF 6 )( p -pymene) 而不是中性 Ru(OAc) 2 ( p -pymene)。合理的催化循环涉及 C-H 活化、马来酰亚胺插入和质子化,以及催化剂的回收和最终产物的形成。计算证实 SbF 6的碎片当第一个反应物1a的羰基中的O原子接近Ru中心时,可以离开Ru中心的配体场形成N-H⋯F氢键,并在最后一步返回Ru配位中心完成催化剂的回收和最终产品的形成。从能量跨度模型推导出决定 TOF 的过渡态 (TDTS) 和中间态 (TDI) 是TS4和INT1,因此在373 K 的温度下活化自由能垒为 28.2 kcal mol -1 。本文讨论了溶剂、导向基团和催化剂类型的影响。
更新日期:2022-10-27
中文翻译:

钌催化 N-甲基苯甲酰胺定向 1,4-加成邻位 C-H 键通过 C-H/C-C 活化与马来酰亚胺的 DFT 研究
密度泛函理论已用于研究钌催化的N-甲基苯甲酰胺通过C-H/C-C 活化将邻位C-H 键定向 1,4-加成到马来酰亚胺,其中活性催化剂被证明是离子型 Ru (OAc)(SbF 6 )( p -pymene) 而不是中性 Ru(OAc) 2 ( p -pymene)。合理的催化循环涉及 C-H 活化、马来酰亚胺插入和质子化,以及催化剂的回收和最终产物的形成。计算证实 SbF 6的碎片当第一个反应物1a的羰基中的O原子接近Ru中心时,可以离开Ru中心的配体场形成N-H⋯F氢键,并在最后一步返回Ru配位中心完成催化剂的回收和最终产品的形成。从能量跨度模型推导出决定 TOF 的过渡态 (TDTS) 和中间态 (TDI) 是TS4和INT1,因此在373 K 的温度下活化自由能垒为 28.2 kcal mol -1 。本文讨论了溶剂、导向基团和催化剂类型的影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号