当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium-Catalyzed Asymmetric Cascade Allylation/Retro-Claisen Reaction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-20 , DOI: 10.1021/jacs.2c08811 Zhi-Yuan Yi 1 , Lu Xiao 1 , Xin Chang 1 , Xiu-Qin Dong 1 , Chun-Jiang Wang 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-20 , DOI: 10.1021/jacs.2c08811 Zhi-Yuan Yi 1 , Lu Xiao 1 , Xin Chang 1 , Xiu-Qin Dong 1 , Chun-Jiang Wang 1, 2
Affiliation
|
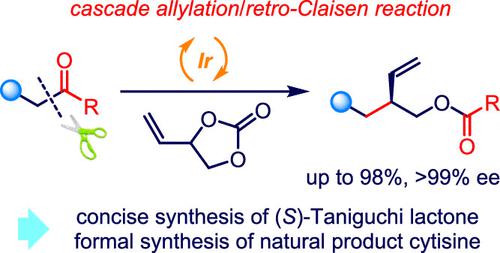
An enantiomerically enriched 3-hydroxymethyl pentenal unit is one of the key structural cores in plenty of natural products and drug candidates with significant biological activities. However, very few synthetic methodologies for the facile construction of the related skeletons have been reported to date. Herein, an elegant iridium-catalyzed asymmetric cascade allylation/retro-Claisen reaction of readily available β-diketones with VEC was successfully developed, and a wide range of functionalized chiral 3-hydroxymethyl pentenal derivatives could be prepared in good yields with excellent enantioselectivities. Various 1,3-diketones and functionalized ketones containing different electron-withdrawing groups on the β-position were well tolerated as outstanding partners with high reactivity and excellent regio-/chemo-/enantioselectivity. The synthetic utility of product chiral 3-hydroxymethyl pentenal derivatives was well shown through gram-scale transformation, hydrogenation, cyclopropanation, hydroboration, and olefin metathesis. Moreover, this elegant protocol demonstrated synthetic applications in the concise synthesis of synthetically useful chiral building block (S)-Taniguchi lactone and the formal synthesis of natural product cytisine. A rational reaction pathway was proposed based on the experimental results and control experiments.
中文翻译:

铱催化的不对称级联烯丙基化/逆克莱森反应
对映体富集的 3-羟甲基戊烯醛单元是许多具有重要生物活性的天然产物和候选药物的关键结构核心之一。然而,迄今为止,很少有关于简单构建相关骨架的合成方法的报道。在此,成功开发了容易获得的 β-二酮与 VEC 的铱催化不对称级联烯丙基化/逆克莱森反应,并且可以以良好的收率和优异的对映选择性制备多种功能化的手性 3-羟甲基戊烯醛衍生物。在 β 位上含有不同吸电子基团的各种 1,3-二酮和功能化酮作为具有高反应性和出色的区域/化学/对映选择性的优秀合作伙伴,具有良好的耐受性。通过克级转化、氢化、环丙烷化、硼氢化和烯烃复分解,很好地展示了产品手性 3-羟甲基戊烯醛衍生物的合成效用。此外,这个优雅的协议展示了在合成有用的手性构建块的简明合成中的合成应用(S )-谷口内酯与天然产物金雀花碱的正式合成。根据实验结果和对照实验提出了合理的反应途径。
更新日期:2022-10-20
中文翻译:

铱催化的不对称级联烯丙基化/逆克莱森反应
对映体富集的 3-羟甲基戊烯醛单元是许多具有重要生物活性的天然产物和候选药物的关键结构核心之一。然而,迄今为止,很少有关于简单构建相关骨架的合成方法的报道。在此,成功开发了容易获得的 β-二酮与 VEC 的铱催化不对称级联烯丙基化/逆克莱森反应,并且可以以良好的收率和优异的对映选择性制备多种功能化的手性 3-羟甲基戊烯醛衍生物。在 β 位上含有不同吸电子基团的各种 1,3-二酮和功能化酮作为具有高反应性和出色的区域/化学/对映选择性的优秀合作伙伴,具有良好的耐受性。通过克级转化、氢化、环丙烷化、硼氢化和烯烃复分解,很好地展示了产品手性 3-羟甲基戊烯醛衍生物的合成效用。此外,这个优雅的协议展示了在合成有用的手性构建块的简明合成中的合成应用(S )-谷口内酯与天然产物金雀花碱的正式合成。根据实验结果和对照实验提出了合理的反应途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号