Chinese Journal of Catalysis ( IF 15.7 ) Pub Date : 2022-10-20 , DOI: 10.1016/s1872-2067(22)64128-7 Ziyi Jiang , Youcheng Hu , Jun Huang , ShengLi Chen
|
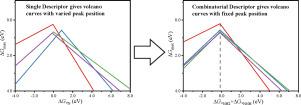
Though touted as a potential way to realize clean ammonia synthesis, electrochemical ammonia synthesis is currently limited by its catalytic efficiency. Great effort has been made to find catalysts with improved activity toward electrochemical nitrogen reduction reaction (eNRR). Rational screening of catalysts can be facilitated using the volcano relationship between catalytic activity and adsorption energy of an intermediate, namely, the activity descriptor. In this work, we propose ΔG*NH2+ΔG*NNH as a combinatorial descriptor, which shows better predictive power than traditional descriptors using the adsorption free energies of single intermediates. The volcano plots based on the combinatorial descriptor exhibits peak activity fixedly at the descriptor value corresponding to the formation free energy of NH3, regardless of the catalyst types; while the descriptor values correspond to the top activities for eNRR on volcano plots based on single descriptors usually vary with the types of catalysts.
中文翻译:

电化学氮还原反应火山关系的组合描述符
尽管被吹捧为实现清洁氨合成的潜在方法,但电化学氨合成目前受到其催化效率的限制。已经做出了巨大的努力来寻找对电化学氮还原反应(eNRR)具有改进活性的催化剂。利用催化活性和中间体吸附能之间的火山关系,即活性描述符,可以促进催化剂的合理筛选。在这项工作中,我们提出 Δ G *NH2 +Δ G *NNH作为组合描述符,它比使用单个中间体的吸附自由能的传统描述符显示出更好的预测能力。无论催化剂类型如何,基于组合描述符的火山图均在对应于 NH 3的形成自由能的描述符值处固定显示峰值活性;而描述符值对应于基于单个描述符的火山图上 eNRR 的最高活性通常随催化剂的类型而变化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号