当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Free Cascade 1,4-Conjugate Addition/Selective Annulation Strategy for Divergent Synthesis of Polysubstituted Imidazoles and 4-Alkenylquinazolines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-10-19 , DOI: 10.1002/adsc.202200905 Qiang Tang 1 , Keke Xu 1 , Xinwei He 1 , Yongjia Shang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-10-19 , DOI: 10.1002/adsc.202200905 Qiang Tang 1 , Keke Xu 1 , Xinwei He 1 , Yongjia Shang 1
Affiliation
|
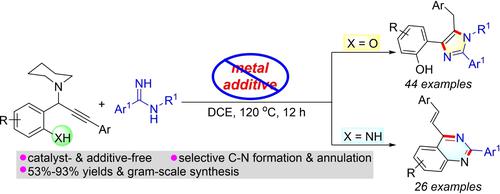
A metal-free cascade annulation of propagylamines with amidines for divergent synthesis of polysubstituted imidazoles and 4-alkenylquinazolines has been developed. The reaction is believed to proceed by sequential 1,4-conjugate addition of amidines to propargylamines, 5-exo-dig annulation/aromatization to form the polysubstituted imidazoles, and nucleophilic addition/elimination/aromatization to quinazoline skeleton. In this reaction, a broad range of electron-rich, electron-neutral, and electron-deficient propagylamines react well with amidines to give polysubstituted imidazoles and 4-alkenylquinazolines in 53–93% yields. Moreover, the utility of this method was showcased by gram-scale experiments and synthetic transformations of the product.
中文翻译:

多取代咪唑和 4-烯基喹唑啉的发散合成的无金属级联 1,4-共轭加成/选择性成环策略
已经开发了丙胺与脒的无金属级联环化,用于多取代咪唑和 4-烯基喹唑啉的发散合成。据信该反应是通过将脒顺序地 1,4-共轭加成到炔丙基胺、5-外环化/芳构化以形成多取代咪唑以及亲核加成/消除/芳构化到喹唑啉骨架来进行的。在该反应中,范围广泛的富电子、电子中性和缺电子丙胺与脒反应良好,以 53-93% 的收率生成多取代咪唑和 4-烯基喹唑啉。此外,该方法的效用已通过克级实验和产物的合成转化得到展示。
更新日期:2022-10-19
中文翻译:

多取代咪唑和 4-烯基喹唑啉的发散合成的无金属级联 1,4-共轭加成/选择性成环策略
已经开发了丙胺与脒的无金属级联环化,用于多取代咪唑和 4-烯基喹唑啉的发散合成。据信该反应是通过将脒顺序地 1,4-共轭加成到炔丙基胺、5-外环化/芳构化以形成多取代咪唑以及亲核加成/消除/芳构化到喹唑啉骨架来进行的。在该反应中,范围广泛的富电子、电子中性和缺电子丙胺与脒反应良好,以 53-93% 的收率生成多取代咪唑和 4-烯基喹唑啉。此外,该方法的效用已通过克级实验和产物的合成转化得到展示。











































 京公网安备 11010802027423号
京公网安备 11010802027423号