当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Intermolecular Dual Amino-Trifluoromethylation of Alkenes by High-Valent Cu(III)−CF3 Compounds
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-10-17 , DOI: 10.1002/adsc.202200945 Ning Chen 1 , Song-Lin Zhang 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-10-17 , DOI: 10.1002/adsc.202200945 Ning Chen 1 , Song-Lin Zhang 2
Affiliation
|
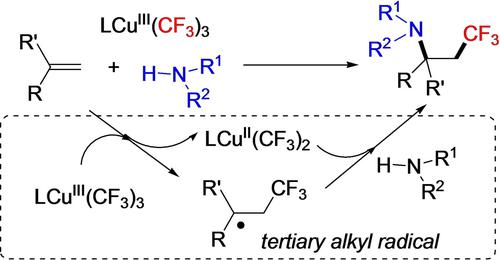
A selective intermolecular amino-trifluoromethylation of alkenes is developed to produce a range of biologically active β-trifluoromethyl amines. This method exploits a crucial high-valent Cu(III)−CF3 complex as a combined source of CF3 radical and Cu(II) oxidant through homolytic Cu(III)−CF3 bond cleavage. This unique mode of CF3 radical generation is distinct from current dominant methods that rely largely on photoredox catalysis or the use of sacrificing radical initiator. This represents a significant advantage in that it greatly simplifies the reaction conditions, brings about operational convenience, and is beneficial to suppress various side reactions under otherwise more complicated conditions. A tertiary alkyl radical generated from addition of CF3 radical to alkenes is proposed to react with amines to enable C−N formation with the assistance of in-situ Cu(II) oxidant. This reaction shows good chemo- and regio-selectivity, suppressing a number of potential side reactions. The free manipulation of readily available Cu(III)−CF3 compound and feedstock compounds of alkenes and anilines greatly broadens the reaction scope. The good chemo- and regioselectivity, and operational convenience make this reaction attractive from the synthetic viewpoint. The reactivity of high-valent Cu(III)−CF3 compounds disclosed in this study may be exploited to develop other interesting reactions, and to enrich the currently underdeveloped Cu(III) chemistry.
中文翻译:

高价 Cu(III)−CF3 化合物选择性分子间双氨基-三氟甲基化烯烃
开发了烯烃的选择性分子间氨基-三氟甲基化,以产生一系列具有生物活性的 β-三氟甲基胺。该方法利用关键的高价 Cu(III)-CF 3络合物作为 CF 3自由基和 Cu(II) 氧化剂的组合来源,通过均裂 Cu(III)-CF 3键断裂。CF 3的这种独特模式自由基生成不同于目前主要依赖光氧化还原催化或使用牺牲自由基引发剂的主要方法。这是一个显着的优势,它大大简化了反应条件,带来了操作上的便利,有利于在其他更复杂的条件下抑制各种副反应。在原位 Cu(II) 氧化剂的帮助下,CF 3自由基与烯烃加成生成的叔烷基自由基与胺反应,从而能够形成 C-N。该反应显示出良好的化学和区域选择性,抑制了许多潜在的副反应。自由操作现成的 Cu(III)−CF 3烯烃和苯胺的化合物和原料化合物大大拓宽了反应范围。良好的化学选择性和区域选择性以及操作便利性使该反应从合成的角度来看具有吸引力。本研究中公开的高价 Cu(III)-CF 3化合物的反应性可用于开发其他有趣的反应,并丰富目前未开发的 Cu(III) 化学。
更新日期:2022-10-17
中文翻译:

高价 Cu(III)−CF3 化合物选择性分子间双氨基-三氟甲基化烯烃
开发了烯烃的选择性分子间氨基-三氟甲基化,以产生一系列具有生物活性的 β-三氟甲基胺。该方法利用关键的高价 Cu(III)-CF 3络合物作为 CF 3自由基和 Cu(II) 氧化剂的组合来源,通过均裂 Cu(III)-CF 3键断裂。CF 3的这种独特模式自由基生成不同于目前主要依赖光氧化还原催化或使用牺牲自由基引发剂的主要方法。这是一个显着的优势,它大大简化了反应条件,带来了操作上的便利,有利于在其他更复杂的条件下抑制各种副反应。在原位 Cu(II) 氧化剂的帮助下,CF 3自由基与烯烃加成生成的叔烷基自由基与胺反应,从而能够形成 C-N。该反应显示出良好的化学和区域选择性,抑制了许多潜在的副反应。自由操作现成的 Cu(III)−CF 3烯烃和苯胺的化合物和原料化合物大大拓宽了反应范围。良好的化学选择性和区域选择性以及操作便利性使该反应从合成的角度来看具有吸引力。本研究中公开的高价 Cu(III)-CF 3化合物的反应性可用于开发其他有趣的反应,并丰富目前未开发的 Cu(III) 化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号