当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ethanol in Aqueous Solution Studied by Microjet Photoelectron Spectroscopy and Theory
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-10-17 , DOI: 10.1021/acs.accounts.2c00471 Hans Ågren 1 , Olle Björneholm 1 , Gunnar Öhrwall 2 , Vincenzo Carravetta 3 , Arnaldo Naves de Brito 4
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-10-17 , DOI: 10.1021/acs.accounts.2c00471 Hans Ågren 1 , Olle Björneholm 1 , Gunnar Öhrwall 2 , Vincenzo Carravetta 3 , Arnaldo Naves de Brito 4
Affiliation
|
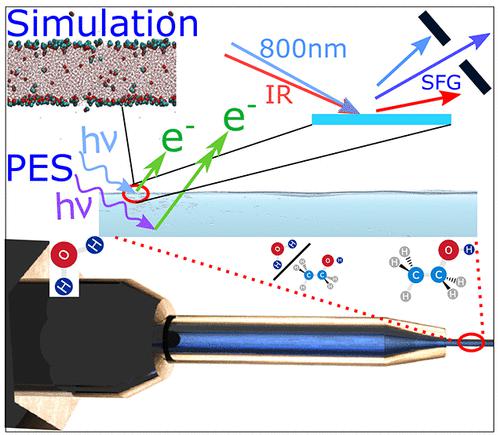
By combining results and analysis from cylindrical microjet photoelectron spectroscopy (cMJ-PES) and theoretical simulations, we unravel the microscopic properties of ethanol–water solutions with respect to structure and intermolecular bonding patterns following the full concentration scale from 0 to 100% ethanol content. In particular, we highlight the salient differences between bulk and surface. Like for the pure water and alcohol constituents, alcohol–water mixtures have attracted much interest in applications of X-ray spectroscopies owing to their potential of combining electronic and geometric structure probing. The water mixtures of the two simplest alcohols, methanol and ethanol, have generated particular attention due to their delicate hydrogen bonding networks that underlie their structural and thermodynamic properties. Macroscopically ethanol–water seems to mix very well, however microscopically this is not true. The aberrant thermodynamics of water–alcohol mixtures have been suggested to be caused by energy differences of hydrogen bonding between water–water, alcohol–alcohol and alcohol–water molecules. These networks may perturb the local character of the interaction between X-rays and matter, calling for analysis that go beyond the normally applied local selection and building block rules and that can combine the effects of light–matter, intra- and intermolecular interactions. However, despite decades of ongoing research there are still controversies of the precise nature of hydrogen bonding networks that underlie the mixing of these simple molecules. Our combined analysis indicates that at low concentration ethanol molecules form a film at the surface since ethanol at the surface can expose its hydrophobic part to the vacuum retaining its two (or three) possible hydrogen bonds, while water at the surface cannot retain all its four possible hydrogen bonds. Thus, ethanol at the surface becomes energetically favorable. Ethanol molecules show a tilting angle variation of the C–C axis with respect to the surface normal as large as 60° at very low concentration. In bulk, around ca. ten %, the ethanol oxygen atoms tend to make a third acceptor hydrogen bond to water molecules. At ca. 20 %, there is a U-shaped change in the CH3 to CH2OH binding energy (BE) shift indicating the presence of ring-like agglomerates called clathrate structures. At the surface, between 5 and 25%, ethanol forms a closely packed layer with the smallest C–C tilting angle variation down to ∼20°. Above 25% and below the azeotrope at the surface, ethanol shows an increase in the tilting angle variation, while at very high ethanol concentrations water tends to move to the surface so giving a microscopic explanation of the azeotrope effect. This migration is connected to the presence of longer (shorter) ethanol chains in the bulk (surface). A brief comparison with discussions and predictions from other spectroscopic techniques is also given. We emphasize the execution of an integrated approach that combines molecular structural dynamics with quantum predictions of the core electronic chemical shift, so establishing a protocol with considerable interpretative as well as predictive power for cMJ-PES measurements. We believe that this protocol can valorize cMJ-PES for studies of properties of other alcohol mixtures as well as of binary solutions in general.
中文翻译:

微射流光电子能谱与理论研究水溶液中的乙醇
通过结合圆柱形微射流光电子能谱 (cMJ-PES) 和理论模拟的结果和分析,我们揭示了乙醇-水溶液在结构和分子间键合模式方面的微观特性,遵循从 0 到 100% 乙醇含量的全浓度范围。特别是,我们强调了体积和表面之间的显着差异。与纯水和酒精成分一样,酒精-水混合物因其结合电子和几何结构探测的潜力而在 X 射线光谱学的应用中引起了极大的兴趣。两种最简单的醇(甲醇和乙醇)的水混合物由于其脆弱的氢键网络而受到特别关注,这些氢键网络是其结构和热力学性质的基础。宏观上乙醇-水似乎混合得很好,但在微观上却并非如此。水-醇混合物的异常热力学被认为是由水-水、醇-醇和醇-水分子之间氢键的能量差异引起的。这些网络可能会扰乱 X 射线与物质之间相互作用的局部特征,需要进行超出通常应用的局部选择和构建块规则的分析,并且可以结合光与物质、分子内和分子间相互作用的影响。然而,尽管进行了数十年的研究,但对于构成这些简单分子混合基础的氢键网络的确切性质,仍存在争议。我们的综合分析表明,在低浓度乙醇分子在表面形成薄膜,因为表面的乙醇可以将其疏水部分暴露在真空中,保留其两个(或三个)可能的氢键,而表面的水不能保留其所有四个可能的氢键。因此,表面的乙醇在能量上变得有利。乙醇分子在非常低的浓度下显示出 C-C 轴相对于表面法线的倾斜角变化高达 60°。散装,大约 ca。百分之十,乙醇氧原子倾向于使第三个受体与水分子形成氢键。大约 20%,CH呈U型变化 而表面的水不能保留所有四个可能的氢键。因此,表面的乙醇在能量上变得有利。乙醇分子在非常低的浓度下显示出 C-C 轴相对于表面法线的倾斜角变化高达 60°。散装,大约 ca。百分之十,乙醇氧原子倾向于使第三个受体与水分子形成氢键。大约 20%,CH呈U型变化 而表面的水不能保留所有四个可能的氢键。因此,表面的乙醇在能量上变得有利。乙醇分子在非常低的浓度下显示出 C-C 轴相对于表面法线的倾斜角变化高达 60°。散装,大约 ca。百分之十,乙醇氧原子倾向于使第三个受体与水分子形成氢键。大约 20%,CH呈U型变化3到通道2OH 结合能 (BE) 变化表明存在称为笼形结构的环状团聚体。在表面,5% 到 25% 之间的乙醇形成一个紧密堆积的层,最小的 C-C 倾斜角变化低至 ~20°。高于 25% 和低于表面的共沸物,乙醇显示出倾斜角变化的增加,而在非常高的乙醇浓度下,水倾向于移动到表面,因此给出共沸物效应的微观解释。这种迁移与本体(表面)中较长(较短)的乙醇链的存在有关。还给出了与其他光谱技术的讨论和预测的简要比较。我们强调执行将分子结构动力学与核心电子化学位移的量子预测相结合的综合方法,因此,为 cMJ-PES 测量建立一个具有相当大的解释和预测能力的协议。我们相信,该协议可以使 cMJ-PES 稳定下来,用于研究其他酒精混合物以及一般二元溶液的特性。
更新日期:2022-10-17
中文翻译:

微射流光电子能谱与理论研究水溶液中的乙醇
通过结合圆柱形微射流光电子能谱 (cMJ-PES) 和理论模拟的结果和分析,我们揭示了乙醇-水溶液在结构和分子间键合模式方面的微观特性,遵循从 0 到 100% 乙醇含量的全浓度范围。特别是,我们强调了体积和表面之间的显着差异。与纯水和酒精成分一样,酒精-水混合物因其结合电子和几何结构探测的潜力而在 X 射线光谱学的应用中引起了极大的兴趣。两种最简单的醇(甲醇和乙醇)的水混合物由于其脆弱的氢键网络而受到特别关注,这些氢键网络是其结构和热力学性质的基础。宏观上乙醇-水似乎混合得很好,但在微观上却并非如此。水-醇混合物的异常热力学被认为是由水-水、醇-醇和醇-水分子之间氢键的能量差异引起的。这些网络可能会扰乱 X 射线与物质之间相互作用的局部特征,需要进行超出通常应用的局部选择和构建块规则的分析,并且可以结合光与物质、分子内和分子间相互作用的影响。然而,尽管进行了数十年的研究,但对于构成这些简单分子混合基础的氢键网络的确切性质,仍存在争议。我们的综合分析表明,在低浓度乙醇分子在表面形成薄膜,因为表面的乙醇可以将其疏水部分暴露在真空中,保留其两个(或三个)可能的氢键,而表面的水不能保留其所有四个可能的氢键。因此,表面的乙醇在能量上变得有利。乙醇分子在非常低的浓度下显示出 C-C 轴相对于表面法线的倾斜角变化高达 60°。散装,大约 ca。百分之十,乙醇氧原子倾向于使第三个受体与水分子形成氢键。大约 20%,CH呈U型变化 而表面的水不能保留所有四个可能的氢键。因此,表面的乙醇在能量上变得有利。乙醇分子在非常低的浓度下显示出 C-C 轴相对于表面法线的倾斜角变化高达 60°。散装,大约 ca。百分之十,乙醇氧原子倾向于使第三个受体与水分子形成氢键。大约 20%,CH呈U型变化 而表面的水不能保留所有四个可能的氢键。因此,表面的乙醇在能量上变得有利。乙醇分子在非常低的浓度下显示出 C-C 轴相对于表面法线的倾斜角变化高达 60°。散装,大约 ca。百分之十,乙醇氧原子倾向于使第三个受体与水分子形成氢键。大约 20%,CH呈U型变化3到通道2OH 结合能 (BE) 变化表明存在称为笼形结构的环状团聚体。在表面,5% 到 25% 之间的乙醇形成一个紧密堆积的层,最小的 C-C 倾斜角变化低至 ~20°。高于 25% 和低于表面的共沸物,乙醇显示出倾斜角变化的增加,而在非常高的乙醇浓度下,水倾向于移动到表面,因此给出共沸物效应的微观解释。这种迁移与本体(表面)中较长(较短)的乙醇链的存在有关。还给出了与其他光谱技术的讨论和预测的简要比较。我们强调执行将分子结构动力学与核心电子化学位移的量子预测相结合的综合方法,因此,为 cMJ-PES 测量建立一个具有相当大的解释和预测能力的协议。我们相信,该协议可以使 cMJ-PES 稳定下来,用于研究其他酒精混合物以及一般二元溶液的特性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号