Journal of Advanced Research ( IF 11.4 ) Pub Date : 2022-10-12 , DOI: 10.1016/j.jare.2022.10.003 Liping Wang 1 , Dongliang Liang 1 , Yinyin Huang 1 , Yunxin Chen 1 , Xiaocong Yang 1 , Zhijun Huang 1 , Yiqin Jiang 1 , Hanfu Su 1 , Lijing Wang 2 , Janak L Pathak 1 , Linhu Ge 1
|
Introduction
Serum amyloid P component (SAP) regulates the innate immune system and microbial diseases. Periodontitis is an inflammatory oral disease developed by the host immune system's interaction with the dysbiotic oral microbiome, thereby SAP could play a role in periodontitis pathogenicity.
Objectives
To investigate the role of SAP in oral microbiome modulation and peridontitis pathogenicity.
Methods
In this study, wildtype and SAP-knockout (KO) mice were used. Ligature-based periodontitis was developed in mice. Oral microbiome diversity was analyzed by 16 s rRNA sequencing. Macrophages and Porphyromonas gingivalis (P. gingivalis) co-culture system analyzed the effect of SAP in macrophage phagocytosis of P. gingivalis.
Results
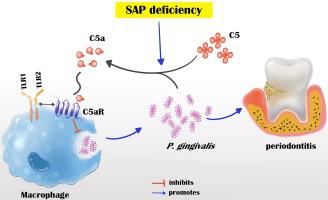
The level of SAP was upregulated in the periodontitis-affected periodontium of humans and mice but not in the liver and blood circulation. Periodontal macrophages were the key source of upregulated SAP in periodontitis. SAP-KO aggravated periodontal inflammation, periodontitis, and a higher number of M1-type inflammatory macrophage infiltration in the periodontium. The oral microbiome of SAP-KO periodontitis mice was altered with a higher abundance of Porphyromonas at the genus level. SAP-KO macrophages showed compromised phagocytosis of P. gingivalis in the co-culture system. Co-culture of SAP-KO macrophages and P. gingivalis induced the C5a expression and exogenous SAP treatment nullified this effect. Exogenous recombinant SAP treatment did not affect P. gingivalis growth and opsonization. PMX205, an antagonist of C5a, treatment robustly enhanced P. gingivalis phagocytosis by SAP-KO macrophages, indicating the involvement of the C5a-C5aR signaling in the compromised P. gingivalis phagocytosis by SAP-KO macrophages.
Conclusion
SAP deficiency aggravates periodontitis possibly via C5a-C5aR signaling-mediated defective macrophage phagocytosis of P. gingivalis. A higher abundance of P. gingivalis during SAP deficiency could promote M1 macrophage polarization and periodontitis. This finding suggests the possible protecting role of elevated levels of periodontal SAP against periodontitis progression.
中文翻译:

SAP 缺乏可能通过 C5a-C5aR 信号介导的巨噬细胞对牙龈卟啉单胞菌的吞噬作用缺陷加重牙周炎
介绍
血清淀粉样蛋白 P 成分 (SAP) 调节先天免疫系统和微生物疾病。牙周炎是一种炎症性口腔疾病,是由宿主免疫系统与失调的口腔微生物相互作用而产生的,因此SAP可能在牙周炎的致病性中发挥作用。
目标
探讨 SAP 在口腔微生物组调节和牙周炎致病性中的作用。
方法
在这项研究中,使用了野生型和 SAP 敲除 (KO) 小鼠。基于结扎的牙周炎是在小鼠中产生的。通过 16 s rRNA 测序分析口腔微生物组多样性。巨噬细胞与牙龈卟啉单胞菌(P.gingivalis)共培养系统分析了SAP对巨噬细胞吞噬牙龈卟啉单胞菌的影响。
结果
SAP 水平在人类和小鼠受牙周炎影响的牙周组织中上调,但在肝脏和血液循环中没有上调。牙周巨噬细胞是牙周炎中 SAP 上调的关键来源。SAP-KO加重牙周炎症,导致牙周炎,牙周内M1型炎性巨噬细胞浸润数量较多。SAP-KO 牙周炎小鼠的口腔微生物组因属水平上卟啉单胞菌丰度的增加而发生改变。SAP-KO 巨噬细胞在共培养系统中表现出对牙龈卟啉单胞菌的吞噬作用受损。SAP-KO 巨噬细胞和牙龈卟啉单胞菌的共培养诱导了 C5a 表达,而外源 SAP 处理消除了这种效应。外源重组 SAP 处理不影响牙龈卟啉单胞菌的生长和调理作用。C5a 拮抗剂 PMX205 治疗可显着增强SAP-KO 巨噬细胞对牙龈卟啉单胞菌的吞噬作用,表明 C5a-C5aR 信号传导参与 SAP-KO 巨噬细胞受损的牙龈卟啉单胞菌吞噬作用。
结论
SAP 缺陷可能通过 C5a-C5aR 信号介导的巨噬细胞吞噬牙龈卟啉单胞菌的缺陷而加重牙周炎。SAP 缺乏期间较高丰度的牙龈卟啉单胞菌可促进 M1 巨噬细胞极化和牙周炎。这一发现表明牙周 SAP 水平升高可能对牙周炎进展具有保护作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号