Journal of Catalysis ( IF 6.5 ) Pub Date : 2022-10-10 , DOI: 10.1016/j.jcat.2022.09.031 Jiayu Li , Minghui Zhu , Yi-Fan Han
|
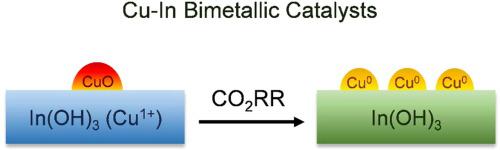
In-based bimetallic catalysts show great potential to reduce CO2 electrochemically. However, the nature of active sites and reaction mechanisms are not fully understood. This study systematically investigates the role of Cu in the Cu-In bimetallic catalysts during the reaction. The partial current densities of formate and CO show a linear correlation with increased Cu content. CuIn3 shows a total current density of 6.40 mA cm−2 and the highest Faradic efficiency for C1 products of 99.3% at −0.7 V vs RHE. A series of characterizations reveal that the Cu1+ species dope into the In(OH)3 lattice for the Cu-In catalysts, while small CuO nanoparticles also form on the surface of catalyst with high Cu contents. The CuO and Cu1+ species are reduced to metallic Cu0, leading to the formation of the Cu0-In(OH)3 interfacial sites. Adding Cu is found to facilitate the adsorption of CO2 and shift the rate-determining step for formate production.
中文翻译:

阐明Cu-In双金属催化剂在CO2RR过程中的结构演变和反应机理
In基双金属催化剂显示出电化学还原CO 2的巨大潜力。然而,活性位点的性质和反应机制尚不完全清楚。本研究系统地研究了Cu在反应过程中在Cu-In双金属催化剂中的作用。甲酸盐和 CO 的部分电流密度与增加的 Cu 含量呈线性相关。CuIn3 的总电流密度为 6.40 mA cm -2,C1 产品的最高法拉第效率在 -0.7 V vs RHE 时为 99.3%。一系列表征表明,Cu 1+物质掺杂到 In(OH) 3中Cu-In 催化剂的晶格,同时在高 Cu 含量的催化剂表面也形成了小的 CuO 纳米颗粒。CuO和Cu 1+物质被还原为金属Cu 0,导致Cu 0 -In(OH) 3界面位点的形成。发现添加Cu有助于CO 2的吸附并改变甲酸盐生产的速率决定步骤。









































 京公网安备 11010802027423号
京公网安备 11010802027423号