当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Yuzurine-type Alkaloid Daphgraciline
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-05 , DOI: 10.1021/jacs.2c09548 Li-Xuan Li 1, 2 , Long Min 1 , Tian-Bing Yao 1 , Shu-Xiao Ji 1 , Chuang Qiao 1 , Pei-Lin Tian 1 , Jianwei Sun 2 , Chuang-Chuang Li 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-05 , DOI: 10.1021/jacs.2c09548 Li-Xuan Li 1, 2 , Long Min 1 , Tian-Bing Yao 1 , Shu-Xiao Ji 1 , Chuang Qiao 1 , Pei-Lin Tian 1 , Jianwei Sun 2 , Chuang-Chuang Li 1, 3
Affiliation
|
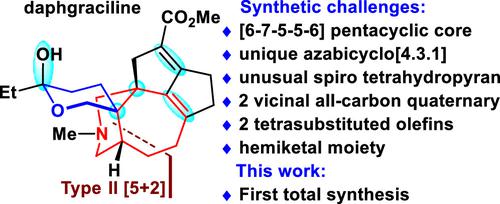
The first total synthesis of daphgraciline has been achieved, which also represents the first example of the synthesis of Daphniphyllum yuzurine-type alkaloids (∼50 members). The unique bridged azabicyclo[4.3.1] ring system in the yuzurine-type subfamily was efficiently and diastereoselectively assembled via a mild type II [5+2] cycloaddition for the first time. The compact tetracyclic [6–7–5–5] skeleton was installed efficiently via an intramolecular Diels–Alder reaction, followed by a benzilic acid-type rearrangement. The synthetically challenging spiro tetrahydropyran moiety in the final product was installed diastereoselectively via a TiIII-mediated reductive epoxide coupling reaction. Potential access to enantioenriched daphgraciline is presented.
中文翻译:

Yuzurine型生物碱Daphgraciline的全合成
实现了daphgraciline的首次全合成,这也代表了Daphniphyllum yuzurine型生物碱(~50个成员)合成的第一个例子。yuzurine 型亚家族中独特的桥联氮杂双环[4.3.1] 环系统首次通过温和的 II 型 [5+2] 环加成反应高效且非对映选择性地组装。紧凑的四环 [6-7-5-5] 骨架通过分子内 Diels-Alder 反应有效安装,然后进行苯甲酸型重排。最终产品中具有合成挑战性的螺四氢吡喃部分通过 Ti III介导的还原性环氧化物偶联反应非对映选择性地安装。介绍了获得对映体富集 daphgraciline 的潜在途径。
更新日期:2022-10-05
中文翻译:

Yuzurine型生物碱Daphgraciline的全合成
实现了daphgraciline的首次全合成,这也代表了Daphniphyllum yuzurine型生物碱(~50个成员)合成的第一个例子。yuzurine 型亚家族中独特的桥联氮杂双环[4.3.1] 环系统首次通过温和的 II 型 [5+2] 环加成反应高效且非对映选择性地组装。紧凑的四环 [6-7-5-5] 骨架通过分子内 Diels-Alder 反应有效安装,然后进行苯甲酸型重排。最终产品中具有合成挑战性的螺四氢吡喃部分通过 Ti III介导的还原性环氧化物偶联反应非对映选择性地安装。介绍了获得对映体富集 daphgraciline 的潜在途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号