Chinese Journal of Catalysis ( IF 15.7 ) Pub Date : 2022-10-04 , DOI: 10.1016/s1872-2067(22)64108-1 Jianjun Zhang , Gaoyuan Yang , Bowen He , Bei Cheng , Youji Li , Guijie Liang , Linxi Wang
|
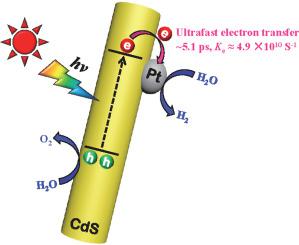
Noble metal cocatalysts have shown great potential in boosting the performance of CdS in photocatalytic water splitting. However, the mechanism and kinetics of electron transfer in noble-metal-decorated CdS during practical hydrogen evolution is not clearly elucidated. Herein, Pt-nanoparticle-decorated CdS nanorods (CdS/Pt) are utilized as the model system to analyze the electron transfer kinetics in CdS/Pt heterojunction. Through femtosecond transient absorption spectroscopy, three dominating exciton quenching pathways are observed and assigned to the trapping of photogenerated electrons at shallow states, recombination of free electrons and trapped holes, and radiative recombination of locally photogenerated electron-hole pairs. The introduction of Pt cocatalyst can release the electrons trapped at the shallow states and construct an ultrafast electron transfer tunnel at the CdS/Pt interface. When CdS/Pt is dispersed in acetonitrile, the lifetime and rate for interfacial electron transfer are respectively calculated to be ~5.5 ps and ~3.5 × 1010 s−1. The CdS/Pt is again dispersed in water to simulate photocatalytic water splitting. The lifetime of the interfacial electron transfer decreases to ~5.1 ps and the electron transfer rate increases to ~4.9 × 1010 s−1, confirming that Pt nanoparticles serve as the main active sites of hydrogen evolution. This work reveals the role of Pt cocatalysts in enhancing the photocatalytic performance of CdS from the perspective of electron transfer kinetics.
中文翻译:

CdS/Pt异质结光催化剂在水分解过程中的电子转移动力学
贵金属助催化剂在提高 CdS 在光催化水分解中的性能方面显示出巨大的潜力。然而,在实际析氢过程中,贵金属装饰的 CdS 中电子转移的机制和动力学尚不清楚。在此,Pt 纳米颗粒装饰的 CdS 纳米棒 (CdS/Pt) 被用作模型系统来分析 CdS/Pt 异质结中的电子转移动力学。通过飞秒瞬态吸收光谱,观察到三种主要的激子猝灭途径,并将其分配给浅态光生电子的俘获、自由电子和俘获空穴的复合以及局部光生电子-空穴对的辐射复合。Pt助催化剂的引入可以释放被困在浅态的电子,在CdS/Pt界面构建超快电子传输隧道。当 CdS/Pt 分散在乙腈中时,界面电子转移的寿命和速率分别计算为 ~5.5 ps 和 ~3.5 × 1010秒-1。CdS/Pt 再次分散在水中以模拟光催化水分解。界面电子转移的寿命降低到~5.1 ps,电子转移速率提高到~4.9 × 10 10 s -1,证实了Pt纳米颗粒是析氢的主要活性位点。这项工作从电子转移动力学的角度揭示了 Pt 助催化剂在提高 CdS 光催化性能方面的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号