Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-10-05 , DOI: 10.1016/j.jhazmat.2022.130126 Kaichao Yang 1 , Ibrahim M Abu-Reesh 2 , Zhen He 1
|
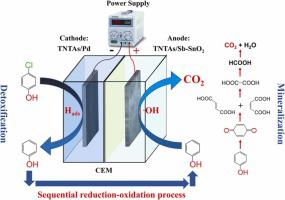
Electrochemical treatment can be an effective approach for degrading recalcitrant organic contaminant because its anode/cathode produces powerful oxidizing/reducing conditions. Herein, through the cooperation of the cathodic reductive and anodic oxidative processes, 4-chlorophenol (4-CP) was successfully degraded in an electrochemical system. TiO2 nanotube arrays (TNTAs)/Sb-SnO2 and TNTAs/Pd were successfully prepared and served as the anode and cathode electrodes, respectively, to generate oxidative (hydroxyl radical, ·OH) and reductive (chemically adsorbed hydrogen, Hads) agents. The sequential reduction-oxidation (SRO) process provided a reasonable degradation pathway that accomplished reductive detoxification in the cathode and oxidative mineralization in the anode. The SRO mode achieved dechlorination efficiency (DE) of 86.9 ± 3.9% and TOC removal efficiency of 64.8 ± 4.2% within 3 h and under a current density of 8 mA cm-2, both of which were significantly higher than those obtained in the sequential oxidation-reduction or the simultaneous redox modes. The increment of current density and reaction time could improve 4-CP degradation performance, but a high current density would decrease the cathode stability and a longer reaction time led to the generation of ClO4-. This study has demonstrated that sequential reduction-oxidation can be an effective and tunable process for degrading recalcitrant organic contaminants.
中文翻译:

在电化学系统中通过协同还原和氧化过程降解 4-氯苯酚
电化学处理可以成为降解顽固性有机污染物的有效方法,因为它的阳极/阴极会产生强大的氧化/还原条件。在此,通过阴极还原和阳极氧化过程的协同作用,4-氯苯酚(4-CP)在电化学系统中被成功降解。成功制备了TiO 2纳米管阵列 (TNTAs)/Sb-SnO 2和 TNTAs/Pd,分别作为阳极和阴极电极,产生氧化性(羟基自由基,·OH)和还原性(化学吸附氢,H ads )) 代理。顺序还原-氧化(SRO)过程提供了一个合理的降解途径,在阴极完成还原解毒和阳极氧化矿化。SRO模式在8 mA cm -2 电流密度下,3 h内脱氯效率(DE)为86.9 ± 3.9%,TOC去除效率为64.8 ± 4.2% ,均显着高于序贯模式。氧化还原或同时氧化还原模式。电流密度和反应时间的增加可以提高 4-CP 的降解性能,但高电流密度会降低正极稳定性,较长的反应时间会导致产生 ClO 4 - . 该研究表明,顺序还原-氧化可以是一种有效且可调节的降解顽固有机污染物的过程。









































 京公网安备 11010802027423号
京公网安备 11010802027423号