JACC: Cardiovascular Interventions ( IF 11.7 ) Pub Date : 2022-10-03 , DOI: 10.1016/j.jcin.2022.07.039 Vijay Kunadian 1 , Usman Baber 2 , Carlo A Pivato 3 , Davide Cao 4 , George Dangas 5 , Samantha Sartori 5 , Zhongjie Zhang 5 , Dominick J Angiolillo 6 , Carlo Briguori 7 , David J Cohen 8 , Timothy Collier 9 , Dariusz Dudek 10 , Michael Gibson 11 , Robert Gil 12 , Kurt Huber 13 , Upendra Kaul 14 , Ran Kornowski 15 , Mitchell W Krucoff 16 , Payam Dehghani 17 , Shamir Mehta 18 , David J Moliterno 19 , E Magnus Ohman 16 , Javier Escaned 20 , Gennaro Sardella 21 , Samin K Sharma 5 , Richard Shlofmitz 22 , Giora Weisz 23 , Bernhard Witzenbichler 24 , Vladimír Džavík 25 , Paul Gurbel 26 , Christian W Hamm 27 , Timothy Henry 28 , Adnan Kastrati 29 , Steven O Marx 30 , Keith Oldroyd 31 , P Gabriel Steg 32 , Stuart Pocock 9 , Roxana Mehran 5
|
Background
There is a paucity of data regarding the safety and efficacy of different antiplatelet regimens according to standardized body mass index (BMI) categories.
Objectives
The aim of this study was to investigate bleeding and ischemic outcomes according to BMI in the TWILIGHT (Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention) trial.
Methods
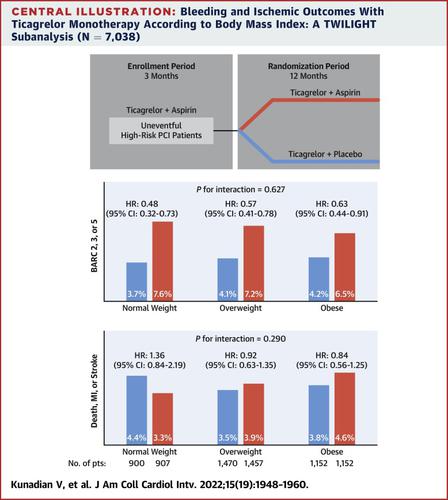
The TWILIGHT trial randomized high-risk patients to ticagrelor plus aspirin or ticagrelor plus placebo at 3 months after percutaneous coronary intervention. In this secondary analysis, patients were stratified by standard BMI categories, as recommended by the European Society of Cardiology Working Group on Thrombosis (normal weight [BMI 18.5-24.99 kg/m2], overweight [BMI 25-29.99 kg/m2], and obese [BMI ≥30 kg/m2]) and by median BMI, as prespecified in the protocol.
Results
Among 7,038 patients randomized and with available BMI, 1,807 (25.7%) were normal weight, 2,927 (41.6%) were overweight, and 2,304 (32.7%) were obese. In normal-weight, overweight, and obese patients, ticagrelor monotherapy, compared with ticagrelor plus aspirin, reduced the primary endpoint of Bleeding Academic Research Consortium type 2, 3, or 5 bleeding (normal weight: HR: 0.48 [95% CI: 0.32-0.73]; overweight: HR: 0.57 [95% CI: 0.41-0.78]; obese: HR: 0.63 [95% CI: 0.44-0.91]; P for interaction = 0.627), without any increase in the composite ischemic endpoint of all-cause death, myocardial infarction, or stroke (normal weight: HR: 1.36 [95% CI: 0.84-2.19]; overweight: HR: 0.92 [95% CI: 0.63-1.35]; obese: HR: 0.84 [95% CI: 0.56-1.25]; P for interaction = 0.290). These findings were consistent with the prespecified analysis by median BMI.
Conclusions
Among high-risk patients undergoing percutaneous coronary intervention, ticagrelor monotherapy, compared with ticagrelor plus aspirin, reduced bleeding events without any increase in ischemic risk across different BMI categories.
中文翻译:

根据体重指数,替格瑞洛单药治疗的出血和缺血结果
背景
根据标准化体重指数 (BMI) 类别,关于不同抗血小板方案的安全性和有效性的数据很少。
目标
本研究的目的是在 TWILIGHT(替格瑞洛联合阿司匹林或单独用于冠脉介入治疗后的高危患者)试验中根据 BMI 调查出血和缺血结果。
方法
TWILIGHT 试验在经皮冠状动脉介入治疗后 3 个月将高危患者随机分配至替格瑞洛联合阿司匹林或替格瑞洛联合安慰剂组。在该次要分析中,按照欧洲心脏病学会血栓形成工作组的建议(正常体重 [BMI 18.5-24.99 kg/m 2 ]、超重 [BMI 25-29.99 kg/m 2 ]) ,按照标准 BMI 类别对患者进行分层, 和肥胖 [BMI ≥ 30 kg/m 2 ]) 和中位数 BMI,如协议中预先规定的那样。
结果
在 7,038 名随机分组且有可用 BMI 的患者中,1,807 名(25.7%)体重正常,2,927 名(41.6%)超重,2,304 名(32.7%)肥胖。在正常体重、超重和肥胖患者中,与替格瑞洛联合阿司匹林相比,替格瑞洛单药治疗可减少出血学术研究联盟 2、3 或 5 型出血的主要终点(正常体重:HR:0.48 [95% CI:0.32 -0.73];超重:HR:0.57 [95% CI:0.41-0.78];肥胖:HR:0.63 [95% CI:0.44-0.91];交互作用P = 0.627),复合缺血性终点无任何增加全因死亡、心肌梗死或中风(正常体重:HR:1.36 [95% CI:0.84-2.19];超重:HR:0.92 [95% CI:0.63-1.35];肥胖:HR:0.84 [95% CI:0.56-1.25];P交互作用 = 0.290)。这些发现与预先设定的中位 BMI 分析一致。
结论
在接受经皮冠状动脉介入治疗的高危患者中,与替格瑞洛联合阿司匹林相比,替格瑞洛单药治疗减少了出血事件,而不同 BMI 类别的缺血风险没有任何增加。









































 京公网安备 11010802027423号
京公网安备 11010802027423号