当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward more potent imidazopyridine inhibitors of Candida albicans Bdf1: Modeling the role of structural waters in selective ligand binding
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-10-03 , DOI: 10.1002/jcc.26997 Yingsheng Zhou 1 , Justin M Overhulse 1 , Nathan J Dupper 1 , Yanchun Guo 1 , Boris A Kashemirov 1 , Kaiyao Wei 2, 3 , Jérôme Govin 3 , Carlo Petosa 2 , Charles E McKenna 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-10-03 , DOI: 10.1002/jcc.26997 Yingsheng Zhou 1 , Justin M Overhulse 1 , Nathan J Dupper 1 , Yanchun Guo 1 , Boris A Kashemirov 1 , Kaiyao Wei 2, 3 , Jérôme Govin 3 , Carlo Petosa 2 , Charles E McKenna 1
Affiliation
|
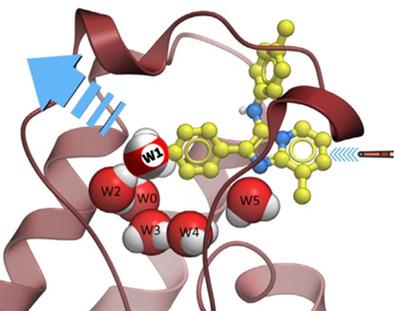
Novel agents to treat invasive fungal infections are urgently needed because the small number of established targets in pathogenic fungi makes the existing drug repertoire particularly vulnerable to the emergence of resistant strains. Recently, we reported that Candida albicans Bdf1, a bromodomain and extra-terminal domain (BET) bromodomain with paired acetyl-lysine (AcK) binding sites (BD1 and BD2) is essential for fungal cell growth and that an imidazopyridine (1) binds to BD2 with selectivity versus both BD1 and human BET bromodomains. Bromodomain binding pockets contain a conserved array of structural waters. Molecular dynamics simulations now reveal that one water molecule is less tightly bound to BD2 than to BD1, explaining the site selectivity of 1. This insight is useful in the performance of ligand docking studies to guide design of more effective Bdf1 inhibitors, as illustrated by the design of 10 new imidazopyridine BD2 ligands 1a–j, for which experimental binding and site selectivity data are presented.
中文翻译:

寻找更有效的白色念珠菌 Bdf1 咪唑并吡啶抑制剂:模拟结构水在选择性配体结合中的作用
迫切需要治疗侵袭性真菌感染的新药物,因为病原真菌中的既定靶点数量较少,使得现有药物特别容易受到耐药菌株的出现的影响。最近,我们报道了白色念珠菌Bdf1,一种溴结构域和额外末端结构域 (BET) 的溴结构域,具有配对的乙酰基赖氨酸 (AcK) 结合位点(BD1 和 BD2),对于真菌细胞生长至关重要,并且咪唑并吡啶 ( 1 ) 与BD2 相对于 BD1 和人 BET 溴结构域具有选择性。布罗莫结构域结合口袋包含一系列保守的结构水。分子动力学模拟现在表明,一个水分子与 BD2 的结合不如与 BD1 的结合紧密,这解释了1的位点选择性。这一见解对于进行配体对接研究非常有用,可指导设计更有效的 Bdf1 抑制剂,如 10 种新的咪唑并吡啶 BD2 配体1a–j的设计所示,并提供了实验结合和位点选择性数据。
更新日期:2022-10-03
中文翻译:

寻找更有效的白色念珠菌 Bdf1 咪唑并吡啶抑制剂:模拟结构水在选择性配体结合中的作用
迫切需要治疗侵袭性真菌感染的新药物,因为病原真菌中的既定靶点数量较少,使得现有药物特别容易受到耐药菌株的出现的影响。最近,我们报道了白色念珠菌Bdf1,一种溴结构域和额外末端结构域 (BET) 的溴结构域,具有配对的乙酰基赖氨酸 (AcK) 结合位点(BD1 和 BD2),对于真菌细胞生长至关重要,并且咪唑并吡啶 ( 1 ) 与BD2 相对于 BD1 和人 BET 溴结构域具有选择性。布罗莫结构域结合口袋包含一系列保守的结构水。分子动力学模拟现在表明,一个水分子与 BD2 的结合不如与 BD1 的结合紧密,这解释了1的位点选择性。这一见解对于进行配体对接研究非常有用,可指导设计更有效的 Bdf1 抑制剂,如 10 种新的咪唑并吡啶 BD2 配体1a–j的设计所示,并提供了实验结合和位点选择性数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号