Ultrasonics Sonochemistry ( IF 8.7 ) Pub Date : 2022-10-03 , DOI: 10.1016/j.ultsonch.2022.106188 Hualin Dong 1 , Peng Wang 1 , Zongyun Yang 1 , Ru Li 1 , Xinglian Xu 1 , Juan Shen 1
|
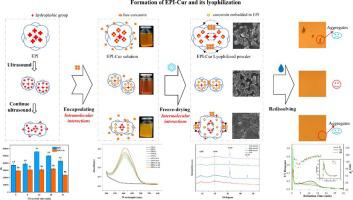
Ultrasound has a recognized ability to modulate the structure and function of proteins. Discovering the influential mechanism of ultrasound on the intramolecular interactions of egg-white protein isolate–curcumin (EPI–Cur) nanoparticles and their intermolecular interaction during freeze drying and redispersion is meaningful. In this study, under the extension of pre-sonication time, the protein solubility, surface hydrophobicity, and curcumin encapsulation rate showed an increasing trend, reaching the highest value at 12 min of treatment. However, the values decreased under the followed extension of ultrasound time. After freeze drying and redispersion were applied, the EPI–Cur sample under 12 min of ultrasound treatment exhibited minimal aggregation degree and loss of curcumin. The retention and loading rates of curcumin in the lyophilized powder reached 96 % and 33.60 mg/g EPI, respectively. However, under excessive ultrasound of >12 min, scanning electron microscopy showed distinct blocky aggregates. Overexposure of the hydrophobic region of the protein triggered protein-mediated hydrophobic aggregation after freeze drying. X-ray diffraction patterns showed the highest crystallinity, indicating that the free curcumin-mediated hydrophobic aggregation during freeze drying was enhanced by the concentration effect and intensified the formation of larger aggregates. This work has practical significance for developing the delivery of hydrophobic active substances. It provides theoretical value for the dynamic dispersity change in protein-hydrophobic active substances during freeze drying and redissolving.
中文翻译:

通过姜黄素-蛋白质组装的超声调控双重提高姜黄素包封效率和冻干复合物分散性
超声波具有公认的调节蛋白质结构和功能的能力。探索超声对蛋清分离蛋白-姜黄素(EPI - Cur)纳米粒子分子内相互作用及其在冷冻干燥和再分散过程中分子间相互作用的影响机制具有重要意义。本研究中,随着预超声处理时间的延长,蛋白溶解度、表面疏水性和姜黄素包封率呈上升趋势,在处理12 min时达到最高值。然而,随着超声时间的延长,这些值会降低。应用冷冻干燥和再分散后,EPI– 超声处理 12 分钟以下的 Cur 样品表现出最小的聚集度和姜黄素损失。冻干粉中姜黄素的保留率和负载率达到 96% 和 33.60 mg/g EPI, 分别。然而,在 >12 分钟的过度超声下,扫描电子显微镜显示明显的块状聚集体。蛋白质疏水区域的过度暴露在冷冻干燥后触发了蛋白质介导的疏水聚集。X 射线衍射图显示出最高的结晶度,表明冷冻干燥过程中游离姜黄素介导的疏水聚集因浓度效应而增强,并加剧了更大聚集体的形成。这项工作对于开发疏水性活性物质的递送具有实际意义。为蛋白质疏水活性物质在冷冻干燥和再溶解过程中的动态分散变化提供了理论价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号