当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selenium Nanowire Formation by Reacting Selenate with Magnetite
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-10-02 , DOI: 10.1021/acs.est.1c08377 Agnieszka Poulain 1 , Alejandro Fernandez-Martinez 1 , Jean-Marc Greneche 2 , Damien Prieur 3 , Andreas C Scheinost 3 , Nicolas Menguy 4 , Sarah Bureau 1 , Valérie Magnin 1 , Nathaniel Findling 1 , Jakub Drnec 5 , Isaac Martens 5 , Marta Mirolo 5 , Laurent Charlet 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-10-02 , DOI: 10.1021/acs.est.1c08377 Agnieszka Poulain 1 , Alejandro Fernandez-Martinez 1 , Jean-Marc Greneche 2 , Damien Prieur 3 , Andreas C Scheinost 3 , Nicolas Menguy 4 , Sarah Bureau 1 , Valérie Magnin 1 , Nathaniel Findling 1 , Jakub Drnec 5 , Isaac Martens 5 , Marta Mirolo 5 , Laurent Charlet 1
Affiliation
|
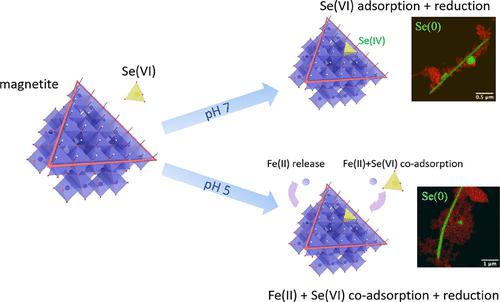
The mobility of 79Se, a fission product of 235U and long-lived radioisotope, is an important parameter in the safety assessment of radioactive nuclear waste disposal systems. Nonradioactive selenium is also an important contaminant of drainage waters from black shale mountains and coal mines. Highly mobile and soluble in its high oxidation states, selenate (Se(VI)O42–) and selenite (Se(IV)O32–) oxyanions can interact with magnetite, a mineral present in anoxic natural environments and in steel corrosion products, thereby being reduced and consequently immobilized by forming low-solubility solids. Here, we investigated the sorption and reduction capacity of synthetic nanomagnetite toward Se(VI) at neutral and acidic pH, under reducing, oxygen-free conditions. The additional presence of Fe(II)aq, released during magnetite dissolution at pH 5, has an effect on the reduction kinetics. X-ray absorption spectroscopy analyses revealed that, at pH 5, trigonal gray Se(0) formed and that sorbed Se(IV) complexes remained on the nanoparticle surface during longer reaction times. The Se(0) nanowires grew during the reaction, which points to a complex transport mechanism of reduced species or to active reduction sites at the tip of the Se(0) nanowires. The concomitant uptake of aqueous Fe(II) and Se(VI) ions is interpreted as a consequence of small pH oscillations that result from the Se(VI) reduction, leading to a re-adsorption of aqueous Fe(II) onto the magnetite, renewing its reducing capacity. This effect is not observed at pH 7, where we observed only the formation of Se(0) with slow kinetics due to the formation of an oxidized maghemite layer. This indicates that the presence of aqueous Fe(II) may be an important factor to be considered when examining the environmental reactivity of magnetite.
中文翻译:

硒酸盐与磁铁矿反应形成硒纳米线
79 Se 是235 U 和长寿命放射性同位素的裂变产物,其迁移率是放射性核废物处置系统安全评估的重要参数。非放射性硒也是黑色页岩山和煤矿排水的重要污染物。在其高氧化态、硒酸盐 (Se( VI )O 4 2– ) 和亚硒酸盐 (Se( IV )O 3 2–) 氧阴离子可以与磁铁矿相互作用,磁铁矿是一种存在于缺氧自然环境和钢铁腐蚀产物中的矿物质,从而被还原并因此通过形成低溶解度固体而被固定。在这里,我们研究了合成纳米磁铁在中性和酸性 pH 值、还原、无氧条件下对 Se(VI) 的吸附和还原能力。Fe( II ) aq的额外存在,在 pH 值为 5 的磁铁矿溶解过程中释放,对还原动力学有影响。X 射线吸收光谱分析表明,在 pH 值为 5 时,形成三角灰色 Se(0),并且吸附的 Se(IV) 配合物在较长的反应时间内保留在纳米颗粒表面。Se(0) 纳米线在反应过程中生长,这表明还原物质的复杂传输机制或Se(0) 纳米线尖端的活性还原位点。水性 Fe(II) 和 Se(VI) 离子的同时吸收被解释为 Se(VI) 还原导致的小 pH 振荡的结果,导致水性 Fe(II) 重新吸附到磁铁矿上,更新其还原能力。在 pH 值为 7 时没有观察到这种效应,因为氧化磁赤铁矿层的形成,我们仅观察到具有缓慢动力学的 Se(0) 的形成。
更新日期:2022-10-02
中文翻译:

硒酸盐与磁铁矿反应形成硒纳米线
79 Se 是235 U 和长寿命放射性同位素的裂变产物,其迁移率是放射性核废物处置系统安全评估的重要参数。非放射性硒也是黑色页岩山和煤矿排水的重要污染物。在其高氧化态、硒酸盐 (Se( VI )O 4 2– ) 和亚硒酸盐 (Se( IV )O 3 2–) 氧阴离子可以与磁铁矿相互作用,磁铁矿是一种存在于缺氧自然环境和钢铁腐蚀产物中的矿物质,从而被还原并因此通过形成低溶解度固体而被固定。在这里,我们研究了合成纳米磁铁在中性和酸性 pH 值、还原、无氧条件下对 Se(VI) 的吸附和还原能力。Fe( II ) aq的额外存在,在 pH 值为 5 的磁铁矿溶解过程中释放,对还原动力学有影响。X 射线吸收光谱分析表明,在 pH 值为 5 时,形成三角灰色 Se(0),并且吸附的 Se(IV) 配合物在较长的反应时间内保留在纳米颗粒表面。Se(0) 纳米线在反应过程中生长,这表明还原物质的复杂传输机制或Se(0) 纳米线尖端的活性还原位点。水性 Fe(II) 和 Se(VI) 离子的同时吸收被解释为 Se(VI) 还原导致的小 pH 振荡的结果,导致水性 Fe(II) 重新吸附到磁铁矿上,更新其还原能力。在 pH 值为 7 时没有观察到这种效应,因为氧化磁赤铁矿层的形成,我们仅观察到具有缓慢动力学的 Se(0) 的形成。







































 京公网安备 11010802027423号
京公网安备 11010802027423号