Chemical Geology ( IF 3.6 ) Pub Date : 2022-10-01 , DOI: 10.1016/j.chemgeo.2022.121147 Elias Niyuhire , Chexin Zhou , Bingbing Hu , Qizheng Cai , Songhu Yuan
|
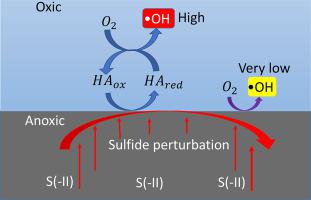
The photo-irradiation of natural organic matter (NOM) is a well-documented pathway of •OH production in surface waters. Using humic acid (HA) as a model compound, we show that the perturbation of NOM-bearing oxic environments by naturally occurring reduced species, sulfide, contributes to •OH production under dark conditions. Sulfide concentration had a more prominent influence on •OH production than HA. When HA was controlled at 16 mM C under oxic and neutral pH conditions, the increase of sulfide concentration from 0 to 5 mM increased the cumulative •OH concentration from <2 to 72.2 μM within 120 min. When sulfide was maintained at 2.5 mM, the increase of HA concentration from 0 to 16 mM C increased the cumulative •OH concentration from <0.6 to 44.8 μM. •OH accumulation showed good consistency with sulfide removal and O2 consumption. O2 consumption increased with increasing HA concentration at a fixed sulfide concentration, and also increased with increasing sulfide concentration at a fixed HA concentration. UV–Vis and FTIR characterizations suggested that the reduced HA moieties resulting from sulfide interaction with HA acted as the redox-active moieties responsible for •OH production. Additionally, 3DEEM fluorescence suggested the reaction of sulfide with oxic HA as a partially reversible reduction-oxidation process with production of labile HA fluorophoric intermediates. Our findings implicate that the perturbation of NOM-bearing oxic environments by sulfide, i.e., from the discharge of anoxic groundwater, springs, seafloor black smokers, and hydrothermal vents, is another important source of •OH in natural environments in addition to photo-irradiation.
中文翻译:

含 NOM 的有氧环境的硫化物扰动诱导暗 •OH 产生
天然有机物 (NOM) 的光辐照是一种在地表水中产生 •OH 的有据可查的途径。使用腐殖酸 (HA) 作为模型化合物,我们表明天然存在的还原物种硫化物对含 NOM 的有氧环境的扰动有助于在黑暗条件下产生 •OH。硫化物浓度对•OH 产生的影响比HA 更显着。当 HA 在有氧和中性 pH 条件下控制在 16 mM C 时,硫化物浓度从 0 增加到 5 mM 会使累积 •OH 浓度在 120 分钟内从 <2 增加到 72.2 μM。当硫化物保持在 2.5 mM 时,HA 浓度从 0 增加到 16 mM C 使累积•OH 浓度从 <0.6 增加到 44.8 μM。消耗。氧2在固定的硫化物浓度下,消耗量随着 HA 浓度的增加而增加,并且在固定的 HA 浓度下随着硫化物浓度的增加而增加。UV-Vis 和FTIR 表征表明,由于硫化物与HA 相互作用而产生的还原HA 部分充当了产生•OH 的氧化还原活性部分。此外,3DEEM 荧光表明硫化物与含氧 HA 的反应是一种部分可逆的还原-氧化过程,会产生不稳定的 HA 荧光中间体。我们的研究结果表明,硫化物对含 NOM 的有氧环境的扰动,即来自缺氧地下水、泉水、海底黑烟囱和热液喷口的排放,是自然环境中除光辐照之外的另一个重要的 •OH 来源.











































 京公网安备 11010802027423号
京公网安备 11010802027423号