Chemical Geology ( IF 3.6 ) Pub Date : 2022-09-29 , DOI: 10.1016/j.chemgeo.2022.121139 C. Baya , P. Le Pape , B. Baptiste , N. Menguy , L. Delbes , M. Morand , M. Rouelle , E. Aubry , G. Ona-Nguema , V. Noël , F. Juillot , G. Morin
|
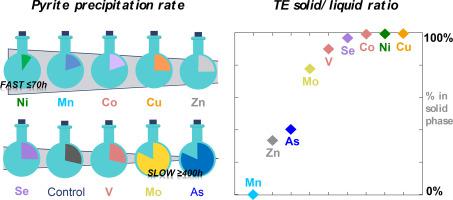
Pyritization links the biogeochemical cycles of Fe, S and C to those of trace elements (TE) such as As, Ni, Cu, Co, Mn, Zn, Se and Mo in reducing environments. The scavenging modes of such impurities in pyrite are parameters of importance for evaluating to which extent pyrite can immobilize TE under diverse (sub)surface conditions. Furthermore, determining the fate of TE during pyritization is a prerequisite to evaluate the ability of pyrite to record the chemical signature of surrounding waters at the time of precipitation. Here, we provide a methodological framework to study the incorporation of TE during a reaction sequence leading to pyrite formation via the polysulfide pathway. Laboratory syntheses were conducted by reacting a FeS precursor with elemental sulfur – both readily obtained by reducing FeCl3 with Na2S in solution under strict anoxia – at ambient temperature and in the presence of aqueous V, Mn, Co, Ni, Cu, Zn, As, Se and Mo in a TE/Fe molar ratio of 0.5 mol%. Within this simplified framework, our experiments aimed at reproducing the early formation of pyrite in water column aggregates/bottom sediments before the late diagenetic aging phases. Solids and liquids were sampled over time during the reaction sequence leading to pyrite formation, up to 3100 h, and further analyzed by ICP-OES, XRD, XRF and STEM-EDXS to obtain information on (1) the kinetics of pyrite formation and (2) the extent of TE incorporation in the solid phase, during and after the complete conversion of FeS to FeS2. Pyrite formation kinetic is observed to be strongly influenced by the identity of the added TE, with the delay before nucleation decreasing in the order Ni >> Mn, Co > Cu, Zn, Se > Control, V >> As, Mo. During the full reaction sequence, i.e. FeS plus S(0) conversion to FeS2, Mn remains in the liquid phase, Ni, Co, V, Cu, and Se quickly precipitate and remain sequestered overtime, Zn and Mo precipitate and are further released into solution, and As is partially incorporated in the solid. Elemental mapping at the microscale using STEM-EDXS show that, except for Mn, the TE are associated with pyrite. Moreover, while TE are homogeneously distributed at the particle scale for Co, Cu, Zn, As, and Se, Ni-pyrite exhibited an enriched core. The solid-liquid partition coefficients of each TE during our pyritization experiments in our simplified model system are calculated and ranks as follows: Cu ≥ Ni ≥ Co > Se > V > Mo >> As > Zn >> Mn. These partitions are discussed in the light of values reported in the literature from natural settings. The important differences observed exemplify the complexity to predict TE pyrite-water partition in sedimentary environments, in particular when considering additional multifactorial processes such as the local biogeochemistry of micro-environments, as well as the partial sequestration of TE by non-sulfide minerals such as authigenic clays and carbonates.
中文翻译:

在环境温度下通过多硫化物途径研究黄铁矿形成过程中 V、Mn、Co、Ni、Cu、Zn、As、Se 和 Mo 的行为和动力学影响的方法框架
黄铁矿化将 Fe、S 和 C 的生物地球化学循环与还原环境中的 As、Ni、Cu、Co、Mn、Zn、Se 和 Mo 等微量元素 (TE) 的生物地球化学循环联系起来。黄铁矿中此类杂质的清除模式是评估黄铁矿在不同(亚)表面条件下可在何种程度上固定 TE 的重要参数。此外,确定黄铁矿化过程中 TE 的命运是评估黄铁矿在降水时记录周围水域化学特征的能力的先决条件。在这里,我们提供了一个方法框架来研究 TE 在通过多硫化物途径形成黄铁矿的反应序列中的掺入。实验室合成是通过将 FeS 前体与元素硫反应进行的——两者都可以通过还原 FeCl2 轻松获得3与 Na 2 S 在严格缺氧条件下的溶液中——在环境温度下,在 TE/Fe 摩尔比为 0.5 mol% 的 V、Mn、Co、Ni、Cu、Zn、As、Se 和 Mo 水溶液中存在。在这个简化的框架内,我们的实验旨在重现晚期成岩老化阶段之前水柱聚集体/底部沉积物中黄铁矿的早期形成。在导致黄铁矿形成的反应过程中,随着时间的推移对固体和液体进行采样,最长可达 3100 小时,并通过 ICP-OES、XRD、XRF 和 STEM-EDXS 进一步分析,以获得有关 (1) 黄铁矿形成动力学和 ( 2) 在 FeS 完全转化为 FeS 2期间和之后,TE 在固相中的结合程度. 观察到黄铁矿形成动力学受所添加 TE 特性的强烈影响,成核前的延迟按 Ni >> Mn、Co > Cu、Zn、Se > Control、V >> As、Mo 的顺序减少。完整的反应序列,即FeS 加 S(0) 转化为 FeS 2, Mn 保留在液相中,Ni、Co、V、Cu 和 Se 迅速沉淀并随着时间的推移保持螯合,Zn 和 Mo 沉淀并进一步释放到溶液中,As 部分结合到固体中。使用 STEM-EDXS 在微观尺度上的元素映射表明,除了 Mn,TE 与黄铁矿有关。此外,虽然 TE 在 Co、Cu、Zn、As 和 Se 的颗粒尺度上均匀分布,但 Ni-黄铁矿表现出富集的核心。在我们简化模型系统的黄铁矿化实验中,每个 TE 的固液分配系数计算如下:Cu ≥ Ni ≥ Co > Se > V > Mo >> As > Zn >> Mn。这些分区是根据自然环境文献中报告的值进行讨论的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号