当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transformation of zinc oxide nanoparticles in freshwater sediments under oxic and anoxic conditions
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2022-09-28 , DOI: 10.1039/d2en00709f Lucie Stetten 1 , Thilo Hofmann 1 , Olivier Proux 2 , Gautier Landrot 3 , Ralf Kaegi 4 , Frank von der Kammer 1
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2022-09-28 , DOI: 10.1039/d2en00709f Lucie Stetten 1 , Thilo Hofmann 1 , Olivier Proux 2 , Gautier Landrot 3 , Ralf Kaegi 4 , Frank von der Kammer 1
Affiliation
|
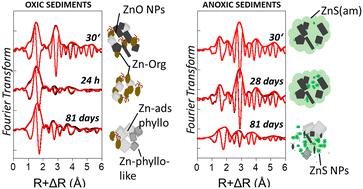
The transformation of zinc oxide nanoparticles (ZnO NPs) has been largely investigated in wastewater treatment plants, recognized as important intermediates before the discharge of NPs into the environment. However, considering direct releases of the pristine ZnO NP forms, additional studies on ZnO NP fate in different environmental compartments are encouraged. In this work, we investigated ZnO NP transformation in lacustrine sediments under defined redox conditions. Using X-ray absorption spectroscopy and wet chemical analyses, we followed ZnO NP and Zn2+ fate over a three-month period in sediments incubated under oxic or anoxic sulfide-rich conditions. Under oxic conditions, ZnO NPs were dissolved within a few hours. By contrast, ZnO NP dissolution under anoxic conditions was much slower, with ∼19% of ZnO NPs remaining at the end of the incubation, together with ∼41% of ZnS, ∼15% of Zn adsorbed onto phyllosilicates and ∼27% of Zn-phyllosilicate-like species. The transient formation of Zn–organic complexes under oxic conditions supports that ZnO NP dissolution is driven by organic compounds, followed by Zn adsorption onto phyllosilicates and the subsequent formation of Zn-layered minerals. Under anoxic conditions, ZnO NP dissolution is inhibited by the precipitation of amorphous ZnS and controlled by the progressive growth of ZnS NPs. These results improve the understanding of ZnO NP transformation in slightly alkaline freshwater sediments and highlight the need to assess NP fate under environmentally relevant conditions.
中文翻译:

氧化锌纳米粒子在有氧和缺氧条件下在淡水沉积物中的转化
氧化锌纳米粒子 (ZnO NPs) 的转化已在废水处理厂中得到广泛研究,在将 NPs 排放到环境中之前,它被认为是重要的中间体。然而,考虑到原始 ZnO NP 形式的直接释放,鼓励对不同环境隔间中的 ZnO NP 命运进行更多研究。在这项工作中,我们研究了在定义的氧化还原条件下湖泊沉积物中的 ZnO NP 转化。使用 X 射线吸收光谱和湿化学分析,我们跟踪了 ZnO NP 和 Zn 2+在富氧或缺氧硫化物条件下培养的沉积物中三个月内的命运。在有氧条件下,ZnO NPs 在几个小时内溶解。相比之下,缺氧条件下的 ZnO NP 溶解要慢得多,在孵育结束时剩余 19% 的 ZnO NP,还有 41% 的 ZnS、15% 的 Zn 吸附在页硅酸盐上和 27% 的 Zn -页硅酸盐样物质。在有氧条件下瞬态形成的 Zn-有机复合物支持 ZnO NP 溶解是由有机化合物驱动的,随后 Zn 吸附到页硅酸盐上,随后形成 Zn 层状矿物。在缺氧条件下,ZnO NP 溶解受到无定形 ZnS 沉淀的抑制,并受 ZnS NPs 逐渐生长的控制。
更新日期:2022-09-28
中文翻译:

氧化锌纳米粒子在有氧和缺氧条件下在淡水沉积物中的转化
氧化锌纳米粒子 (ZnO NPs) 的转化已在废水处理厂中得到广泛研究,在将 NPs 排放到环境中之前,它被认为是重要的中间体。然而,考虑到原始 ZnO NP 形式的直接释放,鼓励对不同环境隔间中的 ZnO NP 命运进行更多研究。在这项工作中,我们研究了在定义的氧化还原条件下湖泊沉积物中的 ZnO NP 转化。使用 X 射线吸收光谱和湿化学分析,我们跟踪了 ZnO NP 和 Zn 2+在富氧或缺氧硫化物条件下培养的沉积物中三个月内的命运。在有氧条件下,ZnO NPs 在几个小时内溶解。相比之下,缺氧条件下的 ZnO NP 溶解要慢得多,在孵育结束时剩余 19% 的 ZnO NP,还有 41% 的 ZnS、15% 的 Zn 吸附在页硅酸盐上和 27% 的 Zn -页硅酸盐样物质。在有氧条件下瞬态形成的 Zn-有机复合物支持 ZnO NP 溶解是由有机化合物驱动的,随后 Zn 吸附到页硅酸盐上,随后形成 Zn 层状矿物。在缺氧条件下,ZnO NP 溶解受到无定形 ZnS 沉淀的抑制,并受 ZnS NPs 逐渐生长的控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号