当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into Chlorobenzene Catalytic Oxidation over Noble Metal Loading {001}-TiO2: The Role of NaBH4 and Subnanometer Ru Undergoing Stable Ru0↔Ru4+ Circulation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-09-27 , DOI: 10.1021/acs.est.2c05981 Bohua Sun 1, 2, 3 , Qianqian Li 1, 2, 3 , Guijin Su 1, 2, 3 , Bowen Meng 1, 2 , Mingge Wu 1, 2, 3 , Qifan Zhang 1, 2, 3 , Jing Meng 1, 2, 3 , Bin Shi 1, 2, 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-09-27 , DOI: 10.1021/acs.est.2c05981 Bohua Sun 1, 2, 3 , Qianqian Li 1, 2, 3 , Guijin Su 1, 2, 3 , Bowen Meng 1, 2 , Mingge Wu 1, 2, 3 , Qifan Zhang 1, 2, 3 , Jing Meng 1, 2, 3 , Bin Shi 1, 2, 3
Affiliation
|
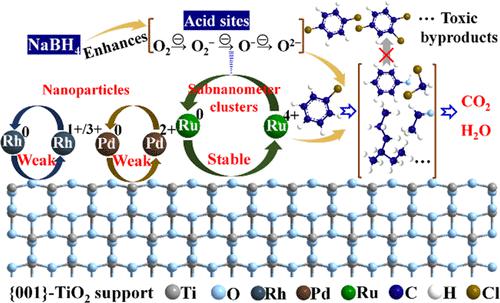
Catalytic combustion of ubiquitous chlorinated volatile organic compounds (CVOCs) encounters bottlenecks regarding catalyst deactivation by chlorine poisoning and generation of toxic polychlorinated byproducts. Herein, Ru, Pd, and Rh were loaded on {001}-TiO2 for thermal catalytic oxidation of chlorobenzene (CB), with Ru/{001}-TiO2 representing superior reactivity, CO2 selectivity, and stability in the 1000 min on-stream test. Interestingly, both acid sites and reactive active oxygen species (ROS) were remarkably promoted via adding NaBH4. But merely enhancing these active sites of the catalyst in CVOC treatment is insufficient. Continuous deep oxidation of CB with effective Cl desorption is also a core issue successfully tackled through the steady Ru0↔Ru4+ circulation. This circulation was facilitated by the observed higher subnanometer Ru dispersion on {001}-TiO2 than the other two noble metals that was supported by single atom stability DFT calculation. Nearly 88 degradation products in off-gas were detected, with Ru/{001}-TiO2 producing the lowest polychlorinated benzene byproducts. An effective and sustainable CB degradation mechanism boosted by the cooperation of NaBH4 enhanced active sites and Ru circulation was proposed accordingly. Insights gained from this study open a new avenue to the rational design of promising catalysts for the treatment of CVOCs.
中文翻译:

对贵金属负载 {001}-TiO2 上氯苯催化氧化的洞察:NaBH4 和亚纳米级 Ru 在稳定 Ru0↔Ru4+ 循环中的作用
无处不在的氯化挥发性有机化合物 (CVOC) 的催化燃烧遇到了因氯中毒导致催化剂失活和产生有毒多氯副产物的瓶颈。在此,Ru、Pd 和 Rh 负载在 {001}-TiO 2上用于氯苯 (CB) 的热催化氧化,Ru/{001}-TiO 2表现出优异的反应活性、CO 2选择性和 1000 分钟内的稳定性在线测试。有趣的是,通过添加 NaBH 4可显着促进酸性位点和活性活性氧 (ROS). 但仅仅提高 CVOC 处理中催化剂的这些活性位点是不够的。CB 的连续深度氧化和有效的 Cl 解吸也是通过稳定的 Ru 0 ↔ Ru 4+循环成功解决的核心问题。在 {001}-TiO 2上观察到比其他两种贵金属更高的亚纳米 Ru 分散促进了这种循环,这一点得到了单原子稳定性 DFT 计算的支持。在废气中检测到近 88 种降解产物,其中 Ru/{001}-TiO 2产生的多氯苯副产物最少。NaBH 4协同促进有效且可持续的CB降解机制相应地提出了增强的活性位点和 Ru 循环。从这项研究中获得的见解为合理设计用于处理 CVOC 的有前途的催化剂开辟了一条新途径。
更新日期:2022-09-27
中文翻译:

对贵金属负载 {001}-TiO2 上氯苯催化氧化的洞察:NaBH4 和亚纳米级 Ru 在稳定 Ru0↔Ru4+ 循环中的作用
无处不在的氯化挥发性有机化合物 (CVOC) 的催化燃烧遇到了因氯中毒导致催化剂失活和产生有毒多氯副产物的瓶颈。在此,Ru、Pd 和 Rh 负载在 {001}-TiO 2上用于氯苯 (CB) 的热催化氧化,Ru/{001}-TiO 2表现出优异的反应活性、CO 2选择性和 1000 分钟内的稳定性在线测试。有趣的是,通过添加 NaBH 4可显着促进酸性位点和活性活性氧 (ROS). 但仅仅提高 CVOC 处理中催化剂的这些活性位点是不够的。CB 的连续深度氧化和有效的 Cl 解吸也是通过稳定的 Ru 0 ↔ Ru 4+循环成功解决的核心问题。在 {001}-TiO 2上观察到比其他两种贵金属更高的亚纳米 Ru 分散促进了这种循环,这一点得到了单原子稳定性 DFT 计算的支持。在废气中检测到近 88 种降解产物,其中 Ru/{001}-TiO 2产生的多氯苯副产物最少。NaBH 4协同促进有效且可持续的CB降解机制相应地提出了增强的活性位点和 Ru 循环。从这项研究中获得的见解为合理设计用于处理 CVOC 的有前途的催化剂开辟了一条新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号