当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Functionalized Pyridinium Salts: A New Chapter for Site-Selective Pyridine C–H Functionalization via Radical-Based Processes under Visible Light Irradiation
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-09-27 , DOI: 10.1021/acs.accounts.2c00530 Myojeong Kim 1, 2 , Yejin Koo 1, 2 , Sungwoo Hong 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-09-27 , DOI: 10.1021/acs.accounts.2c00530 Myojeong Kim 1, 2 , Yejin Koo 1, 2 , Sungwoo Hong 1, 2
Affiliation
|
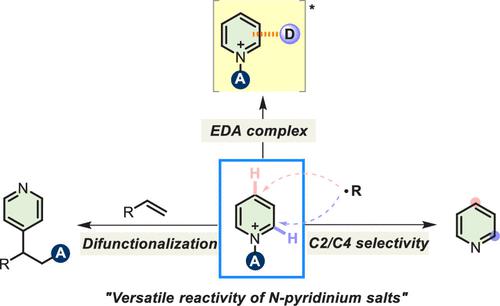
The radical-mediated C–H functionalization of pyridines has attracted considerable attention as a powerful tool in synthetic chemistry for the direct functionalization of the C–H bonds of the pyridine scaffold. Classically, the synthetic methods for functionalized pyridines often involve radical-mediated Minisci-type reactions under strongly acidic conditions. However, the site-selective functionalization of pyridines in unbiased systems has been a long-standing challenge because the pyridine scaffold contains multiple competing reaction sites (C2 vs C4) to intercept free radicals. Therefore, prefunctionalization of the pyridine is required to avoid issues observed with the formation of a mixture of regioisomers and overalkylated side products.
中文翻译:

N-功能化吡啶盐:在可见光照射下通过基于自由基的过程进行位点选择性吡啶 C-H 功能化的新篇章
作为合成化学中吡啶骨架 C-H 键直接功能化的有力工具,自由基介导的吡啶 C-H 功能化引起了相当大的关注。传统上,功能化吡啶的合成方法通常涉及强酸性条件下自由基介导的 Minisci 型反应。然而,吡啶在无偏系统中的位点选择性功能化一直是一个长期存在的挑战,因为吡啶支架包含多个竞争反应位点(C2 与 C4)以拦截自由基。因此,需要对吡啶进行预官能化,以避免在形成区域异构体和过度烷基化副产物的混合物时观察到的问题。
更新日期:2022-09-27
中文翻译:

N-功能化吡啶盐:在可见光照射下通过基于自由基的过程进行位点选择性吡啶 C-H 功能化的新篇章
作为合成化学中吡啶骨架 C-H 键直接功能化的有力工具,自由基介导的吡啶 C-H 功能化引起了相当大的关注。传统上,功能化吡啶的合成方法通常涉及强酸性条件下自由基介导的 Minisci 型反应。然而,吡啶在无偏系统中的位点选择性功能化一直是一个长期存在的挑战,因为吡啶支架包含多个竞争反应位点(C2 与 C4)以拦截自由基。因此,需要对吡啶进行预官能化,以避免在形成区域异构体和过度烷基化副产物的混合物时观察到的问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号