Journal of Vascular and Interventional Radiology ( IF 2.6 ) Pub Date : 2022-09-24 , DOI: 10.1016/j.jvir.2022.09.016 Tushar Garg 1 , Anna J Gong 1 , Adham Khalil 1 , Prateek C Gowda 1 , Robert M Weinstein 1 , Brian P Holly 1 , Clifford R Weiss 1
|
Purpose
To evaluate the racial and ethnic representation of transarterial therapy for hepatocellular carcinoma (HCC) clinical trials in the United States.
Materials and Methods
The ClinicalTrials.gov database was examined to identify all completed studies with transarterial therapies for the management of HCC in the United States and extract information about the observed number of participants for each racial and ethnic group (based on the Office of Management and Budget definitions). The expected number of participants was calculated by multiplying the total number of participants in a trial with the U.S.-population HCC-based proportion for each group. The effects of the study phase, funding source, number of centers involved in the study, and the location of the participating center on racial and ethnic distribution were explored.
Results
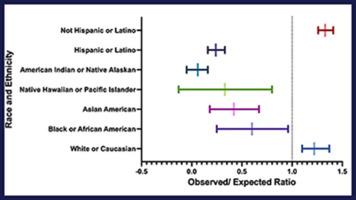
Seventy-nine relevant studies were identified, of which 27 (34.2%) and 18 (22.8%) reported ethnic and race characteristics, respectively. Most study participants were White (81%, 1,591/1,964) by ethnicity and not Hispanic or Latino (93%, 937/1,008) by race. In terms of the observed-to-expected ratios by race and ethnicity in all trials, White and not Hispanic or Latino participants were overrepresented with a ratio of 1.22 (1.10–1.37) and 1.33 (1.26–1.41), respectively, and all other racial and ethnic groups were underrepresented. The enrollment of African Americans and Asian Americans varied by the study phase, and a higher enrollment of African Americans was noted in the National Institutes of Health–funded and multicenter studies (P < .05).
Conclusions
This cross-sectional study demonstrates that in HCC transarterial therapy clinical trials, racial and ethnic minorities were underrepresented and the majority of the studies identified failed to report this demographic information.
中文翻译:

评估经动脉治疗的肝细胞癌临床研究参与者的种族差异
目的
评估美国肝细胞癌 (HCC) 经动脉治疗临床试验的种族和民族代表性。
材料和方法
检查了 ClinicalTrials.gov 数据库,以确定所有已完成的美国 HCC 经动脉治疗管理研究,并提取有关观察到的每个种族和民族参与者人数的信息(基于管理和预算办公室的定义) . 预期参与者人数的计算方法是将试验参与者总数乘以每组美国人群中基于 HCC 的比例。探讨了研究阶段、资金来源、参与研究的中心数量以及参与中心的位置对种族和民族分布的影响。
结果
确定了 79 项相关研究,其中 27 项 (34.2%) 和 18 项 (22.8%) 分别报告了民族和种族特征。大多数研究参与者是白人 (81%, 1,591/1,964),而不是西班牙裔或拉丁裔 (93%, 937/1,008)。就所有试验中种族和族裔的观察到预期比率而言,白人而非西班牙裔或拉丁裔参与者的比例分别为 1.22 (1.10–1.37) 和 1.33 (1.26–1.41),而所有其他种族和族裔群体的代表性不足。非洲裔美国人和亚裔美国人的入学率因研究阶段而异,在美国国立卫生研究院资助的多中心研究中注意到非洲裔美国人的入学率较高 ( P < .05)。
结论
这项横断面研究表明,在 HCC 经动脉治疗临床试验中,种族和少数族裔的人数不足,而且大多数确定的研究都没有报告这一人口统计信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号