当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct Synthesis of α-Aryl-α-Trifluoromethyl Alcohols via Nickel Catalyzed Cross-Electrophile Coupling
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2022-09-26 , DOI: 10.1002/anie.202211732 Lorenzo Lombardi 1, 2 , Alessandro Cerveri 1 , Riccardo Giovanelli 1, 2 , Marta Castiñeira Reis 3 , Carlos Silva López 3 , Giulio Bertuzzi 1, 2 , Marco Bandini 1, 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2022-09-26 , DOI: 10.1002/anie.202211732 Lorenzo Lombardi 1, 2 , Alessandro Cerveri 1 , Riccardo Giovanelli 1, 2 , Marta Castiñeira Reis 3 , Carlos Silva López 3 , Giulio Bertuzzi 1, 2 , Marco Bandini 1, 2
Affiliation
|
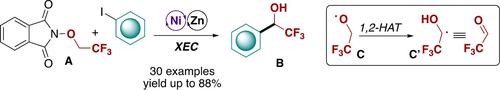
A nickel catalyzed synthesis of α-aryl-α-trifluoromethyl alcohols B is presented that exploits the condensation of iodoarenes and the redox active ether A via cross-electrophile coupling. A mechanistic study was conducted using a comprehensive computational investigation that was also supported by ad hoc control experiments and showed that the key 1,2-HAT (Hydrogen Atom Transfer) event results in the formation of a C-centered radical C′ that mimics the reactivity of trifluoroacetaldehyde.
中文翻译:

镍催化交叉亲电偶联直接合成α-芳基-α-三氟甲基醇
介绍了 α-芳基-α-三氟甲基醇B的镍催化合成,它通过交叉亲电偶联利用碘芳烃和氧化还原活性醚A的缩合。使用全面的计算调查进行了一项机械研究,该调查也得到了特别控制实验的支持,并表明关键的 1,2-HAT(氢原子转移)事件导致形成以 C 为中心的自由基C',它模仿三氟乙醛的反应性。
更新日期:2022-09-26
中文翻译:

镍催化交叉亲电偶联直接合成α-芳基-α-三氟甲基醇
介绍了 α-芳基-α-三氟甲基醇B的镍催化合成,它通过交叉亲电偶联利用碘芳烃和氧化还原活性醚A的缩合。使用全面的计算调查进行了一项机械研究,该调查也得到了特别控制实验的支持,并表明关键的 1,2-HAT(氢原子转移)事件导致形成以 C 为中心的自由基C',它模仿三氟乙醛的反应性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号