当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Determination and Thermodynamic Modeling of Solid–Liquid Equilibria in the Quaternary System NaCl–Na2SO4–NaHCO3–H2O at 323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-26 , DOI: 10.1021/acs.jced.2c00463 Xiaohui Song 1 , Jinqiu Ma 1 , Lingzong Meng 1 , Tianlong Deng 1 , Yafei Guo 1 , Yong Ma 1 , Dan Li 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-26 , DOI: 10.1021/acs.jced.2c00463 Xiaohui Song 1 , Jinqiu Ma 1 , Lingzong Meng 1 , Tianlong Deng 1 , Yafei Guo 1 , Yong Ma 1 , Dan Li 1
Affiliation
|
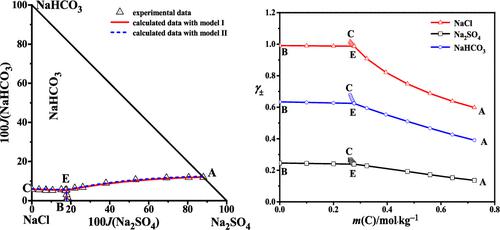
The solubilities, refractive indices, and pH values in the NaCl–Na2SO4–NaHCO3–H2O system at 323.15 K were determined by the isothermal solubility method. The dry-salt diagram of the system consists of three isothermal solubility curves saturated with two salts, one invariant point, and three crystallization regions of NaCl, Na2SO4, and NaHCO3. Two models were applied to predict the solubilities in the system based on the Pitzer model. Model I assumes that there is only one carbon species HCO3– in the solution. Model II supposes that there are three carbon species corresponding to HCO3–, CO32–, CO2(aq), and OH– in the solution. Both the computed solubilities with the two models were basically consistent with experimental results. The concentrations of HCO3–, CO32–, CO2(aq), and OH– were calculated by model II. The dominant form of carbon species is HCO3–, and the concentrations of CO32– and CO2(aq) in the sodium bicarbonate solution can be neglected. Model II can be used to describe the experimental pH values. The mean activity coefficients of NaCl, Na2SO4, and NaHCO3, osmotic coefficients, and water activities were also calculated with the Pitzer model.
中文翻译:

323.15 K 时第四纪体系 NaCl–Na2SO4–NaHCO3–H2O 中固液平衡的溶解度测定和热力学模型
NaCl-Na 2 SO 4 -NaHCO 3 -H 2 O体系在323.15 K下的溶解度、折射率和pH值采用等温溶解度法测定。该体系的干盐图由三个等温溶解度曲线组成,其中两种盐饱和,一个不变点,三个NaCl、Na 2 SO 4和NaHCO 3结晶区。基于Pitzer模型应用了两个模型来预测系统中的溶解度。模型 I 假设溶液中只有一种碳物质 HCO 3 -。模型 II 假设有三种碳物种对应于 HCO 3 -、CO 3 2–、CO 2 (aq) 和 OH –在溶液中。两种模型计算的溶解度与实验结果基本一致。HCO 3 –、CO 3 2–、CO 2 (aq) 和 OH –的浓度由模型 II 计算。碳物种的主要形式是 HCO 3 –,碳酸氢钠溶液中 CO 3 2–和 CO 2 (aq) 的浓度可以忽略不计。模型 II 可用于描述实验 pH 值。NaCl、Na 2 SO 4的平均活度系数, 和 NaHCO 3 , 渗透系数和水分活度也用 Pitzer 模型计算。
更新日期:2022-09-26
中文翻译:

323.15 K 时第四纪体系 NaCl–Na2SO4–NaHCO3–H2O 中固液平衡的溶解度测定和热力学模型
NaCl-Na 2 SO 4 -NaHCO 3 -H 2 O体系在323.15 K下的溶解度、折射率和pH值采用等温溶解度法测定。该体系的干盐图由三个等温溶解度曲线组成,其中两种盐饱和,一个不变点,三个NaCl、Na 2 SO 4和NaHCO 3结晶区。基于Pitzer模型应用了两个模型来预测系统中的溶解度。模型 I 假设溶液中只有一种碳物质 HCO 3 -。模型 II 假设有三种碳物种对应于 HCO 3 -、CO 3 2–、CO 2 (aq) 和 OH –在溶液中。两种模型计算的溶解度与实验结果基本一致。HCO 3 –、CO 3 2–、CO 2 (aq) 和 OH –的浓度由模型 II 计算。碳物种的主要形式是 HCO 3 –,碳酸氢钠溶液中 CO 3 2–和 CO 2 (aq) 的浓度可以忽略不计。模型 II 可用于描述实验 pH 值。NaCl、Na 2 SO 4的平均活度系数, 和 NaHCO 3 , 渗透系数和水分活度也用 Pitzer 模型计算。










































 京公网安备 11010802027423号
京公网安备 11010802027423号