Water Research ( IF 11.4 ) Pub Date : 2022-09-24 , DOI: 10.1016/j.watres.2022.119143 Wenxiao Zheng 1 , Yingkai Chen 1 , Hengyi Fu 1 , Zhang Yan 1 , Zhenchao Lei 1 , Weijian Duan 1 , Chunhua Feng 1
|
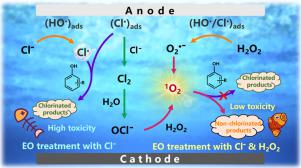
The generation of chlorinated byproducts during the electrochemical oxidation (EO) of Cl−-laden wastewater is a significant concern. We aim to propose a concept of converting reactive species (e.g., reactive chlorines and HO• resulting from electrolysis) into 1O2 via the addition of H2O2, which substantially alleviates chlorinated organic formation. When phenol was used as a model organic compound, the results showed that the H2O2-involving EO system outperformed the H2O2-absent system in terms of higher rate constants (5.95 × 10−2 min−1 vs. 2.97 × 10−2 min−1) and a much lower accumulation of total organic chlorinated products (1.42 mg L−1 vs. 8.18 mg L−1) during a 60 min operation. The rate constants of disappearance of a variety of phenolic compounds were positively correlated with the Hammett constants (σ), suggesting that the reactive species preferred oxidizing phenols with electron-rich groups. After the identification of 1O2 that was abundant in the bulk solution with the use of electron paramagnetic resonance and computational kinetic simulation, the routes of 1O2 generation were revealed. Despite the consensus as to the contribution of reaction between H2O2 and ClO− to 1O2 formation, we conclude that the predominant pathway is through H2O2 reaction with electrogenerated HO• or chlorine radicals (Cl• and Cl2•−) to produce O2•−, followed by self-combination. Density functional theory calculations theoretically showed the difficulty in forming chlorinated byproducts for the 1O2-initiated phenol oxidation in the presence of Cl−, which, by contrast, easily occurred for the Cl•-or HO•-initiated phenol reaction. The experiments run with real coking wastewater containing high-concentration phenols further demonstrated the superiority of the H2O2-involving EO system. The findings imply that this unique method for treating Cl−-laden organic wastewater is expected to be widely adopted for generalizing EO technology for environmental applications.
中文翻译:

在含氯废水中苯酚的电氧化过程中,活性物质转化为 1O2 可显着抑制氯化副产物的形成
在含氯废水的电化学氧化(EO)过程中产生氯化副产物是一个重要的问题。我们旨在提出通过添加H 2 O 2将活性物质(例如,电解产生的活性氯和HO • )转化为1 O 2的概念,这大大减轻了氯化有机物的形成。当苯酚用作模型有机化合物时,结果表明,包含 H 2 O 2的EO 系统在更高的速率常数(5.95 × 10 -2 min -1 与2.97 × 10 -2 min -1相比)和在 60 分钟操作期间总有机氯化产物的积累要低得多(1.42 mg L -1 对8.18 mg L -1 )。多种酚类化合物的消失速率常数与哈米特常数 ( σ ) 呈正相关,表明活性物质更喜欢具有富电子基团的氧化酚。在利用电子顺磁共振和计算动力学模拟鉴定了本体溶液中丰富的1 O 2之后, 1 O 2的路线一代被揭露。尽管对 H 2 O 2和 ClO -之间的反应对1 O 2形成的贡献达成共识,但我们得出的结论是,主要途径是通过 H 2 O 2与电生成的 HO •或氯自由基(Cl •和 Cl 2 • − ) 产生O 2 • −,然后进行自结合。密度泛函理论计算从理论上表明1 O 2难以形成氯化副产物在Cl -存在下-引发的苯酚氧化,相比之下,对于Cl • - 或HO • -引发的苯酚反应很容易发生。使用含有高浓度酚类的真实焦化废水进行的实验进一步证明了包含 H 2 O 2的 EO 系统的优越性。研究结果表明,这种处理含氯有机废水的独特方法有望广泛用于将 EO 技术推广到环境应用中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号