Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2022-09-24 , DOI: 10.1016/j.biopha.2022.113747 Pablo Zubiaur 1 , Laura Figueiredo-Tor 1 , Gonzalo Villapalos-García 1 , Paula Soria-Chacartegui 1 , Marcos Navares-Gómez 1 , Jesús Novalbos 1 , Miriam Matas 1 , Sofía Calleja 1 , Gina Mejía-Abril 1 , Manuel Román 1 , Dolores Ochoa 1 , Francisco Abad-Santos 2
|
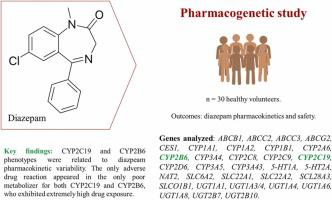
Diazepam is a benzodiazepine (BZD) used worldwide for a variety of conditions. Long-term use of diazepam increases the risk for developing tolerance and dependence and for the occurrence of adverse drug reactions (ADRs). CYP3A4 and CYP2C19 mainly metabolize diazepam and are therefore the primary pharmacogenetic candidate biomarkers. In this work, we aimed to explore the impact of CYP3A4 and CYP2C19 phenotypes and of 99 additional variants in other 31 pharmacogenes (including other CYP, UGT, NAT2 and CES enzymes, ABC and SLC transporters) on diazepam pharmacokinetic variability and safety. 30 healthy volunteers that had participated in a single-dose bioequivalence clinical trial of two diazepam formulations were enrolled in the present candidate gene pharmacogenetic study. CYP2C19 poor metabolizers (PMs) showed an almost 2-fold increase in AUC0-∞/DW compared to rapid (RMs) or normal (NM) metabolizers, and a 1.46-fold increase compared to intermediate metabolizers (IMs). CYP2B6 PMs showed a 2,74-fold higher AUC0-∞/DW compared to RMs, and 2.10-fold compared to NMs (p < 0.007). A dose reduction of 25–50 % may be appropriate for CYP2C19 or CYP2B6 PMs to avoid ADRs, dependence and tolerance. Combined CYP2C19 +CYP2B6 PMs may not use diazepam or sharper dose adjustments (e.g., a dose reduction of 50–70 %) may be advisable. To our knowledge, this is the first work to report a strong relationship between CYP2B6 phenotype and diazepam pharmacokinetics. Additional nominal associations (i.e., 0.007 <p < 0.05) between ABCG2, ABCB1, NAT2 and UGT1A4 polymorphisms and pharmacokinetic variability were observed; further research should elaborate on the clinical relevance of the described associations.
中文翻译:

CYP2C19和CYP2B6表型与地西泮的药代动力学和安全性之间的关联
地西泮是一种苯二氮卓类药物 (BZD),在全球范围内用于各种疾病。长期使用地西泮会增加产生耐受性和依赖性以及发生药物不良反应 (ADR) 的风险。CYP3A4 和 CYP2C19 主要代谢地西泮,因此是主要的药物遗传学候选生物标志物。在这项工作中,我们旨在探索 CYP3A4 和 CYP2C19 表型以及其他 31 种药物基因(包括其他 CYP、UGT、NAT2 和 CES 酶、ABC 和 SLC 转运蛋白)中的 99 种其他变体对地西泮药代动力学变异性和安全性的影响。参加过两种地西泮制剂的单剂量生物等效性临床试验的 30 名健康志愿者参加了本候选基因药物遗传学研究。CYP2C19 弱代谢者 (PM) 的 AUC 增加了近 2 倍与快速 (RMs) 或正常 (NM) 代谢者相比, 0-∞/ DW,与中间代谢者 (IMs) 相比增加 1.46 倍。与 RM 相比, CYP2B6 PM 的 AUC 0-∞ /DW 高 2.74 倍,与 NM 相比高 2.10 倍(p < 0.007)。CYP2C19 或 CYP2B6 PM 的剂量减少 25-50% 可能是合适的,以避免 ADR、依赖性和耐受性。联合使用 CYP2C19 + CYP2B6 PMs 可能不使用地西泮或更剧烈的剂量调整(例如,剂量减少 50-70%)可能是可取的。据我们所知,这是第一项报告 CYP2B6 表型与地西泮药代动力学之间密切关系的工作。ABCG2、ABCB1、NAT2和UGT1A4之间的附加名义关联(即 0.007 <p < 0.05)观察到多态性和药代动力学变异性;进一步的研究应详细说明所述关联的临床相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号