Gastroenterology ( IF 25.7 ) Pub Date : 2022-09-20 , DOI: 10.1053/j.gastro.2022.08.054 C Fiorella Murillo Perez 1 , Holly Fisher 2 , Shaun Hiu 2 , Dorcas Kareithi 2 , Femi Adekunle 3 , Tracy Mayne 3 , Elizabeth Malecha 3 , Erik Ness 3 , Adriaan J van der Meer 4 , Willem J Lammers 4 , Palak J Trivedi 5 , Pier Maria Battezzati 6 , Frederik Nevens 7 , Kris V Kowdley 8 , Tony Bruns 9 , Nora Cazzagon 10 , Annarosa Floreani 10 , Andrew L Mason 11 , Albert Parés 12 , Maria-Carlota Londoño 12 , Pietro Invernizzi 13 , Marco Carbone 14 , Ana Lleo 15 , Marlyn J Mayo 16 , George N Dalekos 17 , Nikolaos K Gatselis 17 , Douglas Thorburn 18 , Xavier Verhelst 19 , Aliya Gulamhusein 1 , Harry L A Janssen 20 , Rachel Smith 21 , Steve Flack 22 , Victoria Mulcahy 22 , Michael Trauner 23 , Christopher L Bowlus 24 , Keith D Lindor 25 , Christophe Corpechot 26 , David Jones 2 , George Mells 27 , Gideon M Hirschfield 1 , James Wason 28 , Bettina E Hansen 29 ,
|
Background & Aims
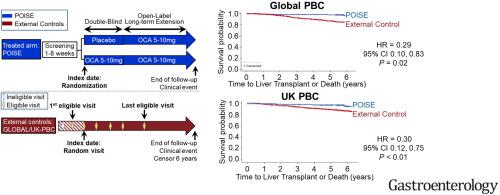
The Primary Biliary Cholangitis (PBC) Obeticholic Acid (OCA) International Study of Efficacy (POISE) randomized, double-blind, placebo-controlled trial demonstrated that OCA reduced biomarkers associated with adverse clinical outcomes (ie, alkaline phosphatase, bilirubin, aspartate aminotransferase, and alanine aminotransferase) in patients with PBC. The objective of this study was to evaluate time to first occurrence of liver transplantation or death in patients with OCA in the POISE trial and open-label extension vs comparable non-OCA–treated external controls.
Methods
Propensity scores were generated for external control patients meeting POISE eligibility criteria from 2 registry studies (Global PBC and UK-PBC) using an index date selected randomly between the first and last date (inclusive) on which eligibility criteria were met. Cox proportional hazards models weighted by inverse probability of treatment assessed time to death or liver transplantation. Additional analyses (Global PBC only) added hepatic decompensation to the composite end point and assessed efficacy in patients with or without cirrhosis.
Results
During the 6-year follow-up, there were 5 deaths or liver transplantations in 209 subjects in the POISE cohort (2.4%), 135 of 1381 patients in the Global PBC control (10.0%), and 281 of 2135 patients in the UK-PBC control (13.2%). The hazard ratios (HRs) for the primary outcome were 0.29 (95% CI, 0.10–0.83) for POISE vs Global PBC and 0.30 (95% CI, 0.12–0.75) for POISE vs UK-PBC. In the Global PBC study, HR was 0.20 (95% CI, 0.03–1.22) for patients with cirrhosis and 0.31 (95% CI, 0.09–1.04) for those without cirrhosis; HR was 0.42 (95% CI, 0.21–0.85) including hepatic decompensation.
Conclusions
Patients treated with OCA in a trial setting had significantly greater transplant-free survival than comparable external control patients.
中文翻译:

与真实世界的外部对照相比,在临床试验环境中接受奥贝胆酸治疗原发性胆汁性胆管炎的患者的无移植生存期更长
背景与目标
原发性胆汁性胆管炎 (PBC) 奥贝胆酸 (OCA) 国际疗效研究 (POISE) 随机、双盲、安慰剂对照试验表明,OCA 减少了与不良临床结果相关的生物标志物(即碱性磷酸酶、胆红素、天冬氨酸氨基转移酶、和谷丙转氨酶)在 PBC 患者中。本研究的目的是评估 POISE 试验和开放标签扩展与可比较的非 OCA 治疗的外部对照相比,OCA 患者首次发生肝移植或死亡的时间。
方法
使用在满足资格标准的第一个和最后一个日期(含)之间随机选择的索引日期,为来自 2 项注册研究(全球 PBC 和 UK-PBC)的符合 POISE 资格标准的外部对照患者生成倾向评分。由治疗的逆概率加权的 Cox 比例风险模型评估了死亡或肝移植的时间。额外的分析(仅限全球 PBC)将肝脏失代偿添加到复合终点,并评估了在有或没有肝硬化患者中的疗效。
结果
在 6 年的随访期间,POISE 队列中的 209 名受试者 (2.4%)、全球 PBC 对照组的 1381 名患者中的 135 名 (10.0%) 和英国的 2135 名患者中的 281 名有 5 名死亡或肝移植-PBC 控制 (13.2%)。POISE 与全球 PBC 的主要结果的风险比 (HR) 为 0.29(95% CI,0.10–0.83),POISE 与 UK-PBC 的主要结果的风险比 (HR) 为 0.30(95% CI,0.12–0.75)。在全球 PBC 研究中,肝硬化患者的 HR 为 0.20(95% CI,0.03-1.22),无肝硬化患者的 HR 为 0.31(95% CI,0.09-1.04);HR 为 0.42(95% CI,0.21–0.85),包括肝脏失代偿。
结论
在试验环境中接受 OCA 治疗的患者的无移植生存期明显高于可比较的外部对照患者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号