当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapor–Liquid Equilibrium for the Binary System of 2-Phenylethanol + 2-Ethylphenol at 50.0, 20.0, and 10.5 kPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-21 , DOI: 10.1021/acs.jced.2c00361 Huisheng Zhao 1 , Yanyang Wu 1 , Bin Wu 1 , Kui Chen 1 , Lijun Ji 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2022-09-21 , DOI: 10.1021/acs.jced.2c00361 Huisheng Zhao 1 , Yanyang Wu 1 , Bin Wu 1 , Kui Chen 1 , Lijun Ji 1
Affiliation
|
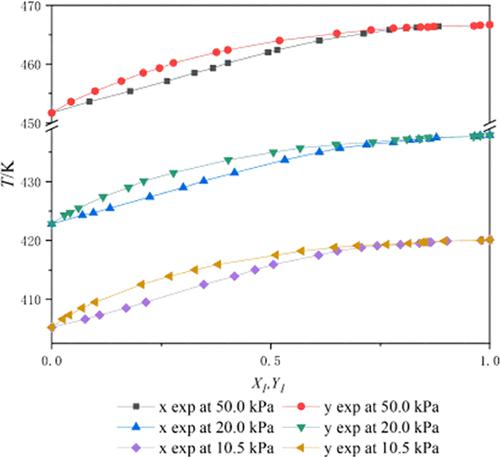
The vapor–liquid equilibrium (VLE) data for binary systems 2-PE and 2-EP were measured at 50.0, 20.0, and 10.5 kPa using a modified Rose equilibrium still. The Redlich–Kister area test and Fredenslund point test were applied to test the thermodynamic consistency of the VLE data, respectively. In addition, experimental data were successfully matched using nonrandom two-liquid (NRTL), Wilson, and Universal Quasi-Chemical (UNIQUAC) activity coefficient models. Binary interaction parameters (BIPs) corresponding to three models were obtained by maximum likelihood target function regression. This paper would help the distillation design of 2-phenylethanol and 2-ethylphenol in industrial applications.
中文翻译:

2-苯乙醇 + 2-乙基苯酚二元体系在 50.0、20.0 和 10.5 kPa 下的汽液平衡
使用改进的 Rose 平衡蒸馏器在 50.0、20.0 和 10.5 kPa 下测量二元系统 2-PE 和 2-EP 的汽液平衡 (VLE) 数据。分别应用 Redlich-Kister 面积检验和 Fredenslund 点检验来检验 VLE 数据的热力学一致性。此外,使用非随机二液 (NRTL)、Wilson 和通用准化学 (UNIQUAC) 活度系数模型成功匹配了实验数据。通过最大似然目标函数回归获得对应于三个模型的二元交互参数(BIP)。本文将有助于工业应用中2-苯基乙醇和2-乙基苯酚的蒸馏设计。
更新日期:2022-09-21
中文翻译:

2-苯乙醇 + 2-乙基苯酚二元体系在 50.0、20.0 和 10.5 kPa 下的汽液平衡
使用改进的 Rose 平衡蒸馏器在 50.0、20.0 和 10.5 kPa 下测量二元系统 2-PE 和 2-EP 的汽液平衡 (VLE) 数据。分别应用 Redlich-Kister 面积检验和 Fredenslund 点检验来检验 VLE 数据的热力学一致性。此外,使用非随机二液 (NRTL)、Wilson 和通用准化学 (UNIQUAC) 活度系数模型成功匹配了实验数据。通过最大似然目标函数回归获得对应于三个模型的二元交互参数(BIP)。本文将有助于工业应用中2-苯基乙醇和2-乙基苯酚的蒸馏设计。









































 京公网安备 11010802027423号
京公网安备 11010802027423号